What family does sodium (Na) belong to? Use your periodic table.
Alkali metals
How does an atom become positively charged? Does it gain or lose electrons?
It loses electrons (so it has more protons than electrons)
In a Bohr model of fluorine, how many electrons are shown?
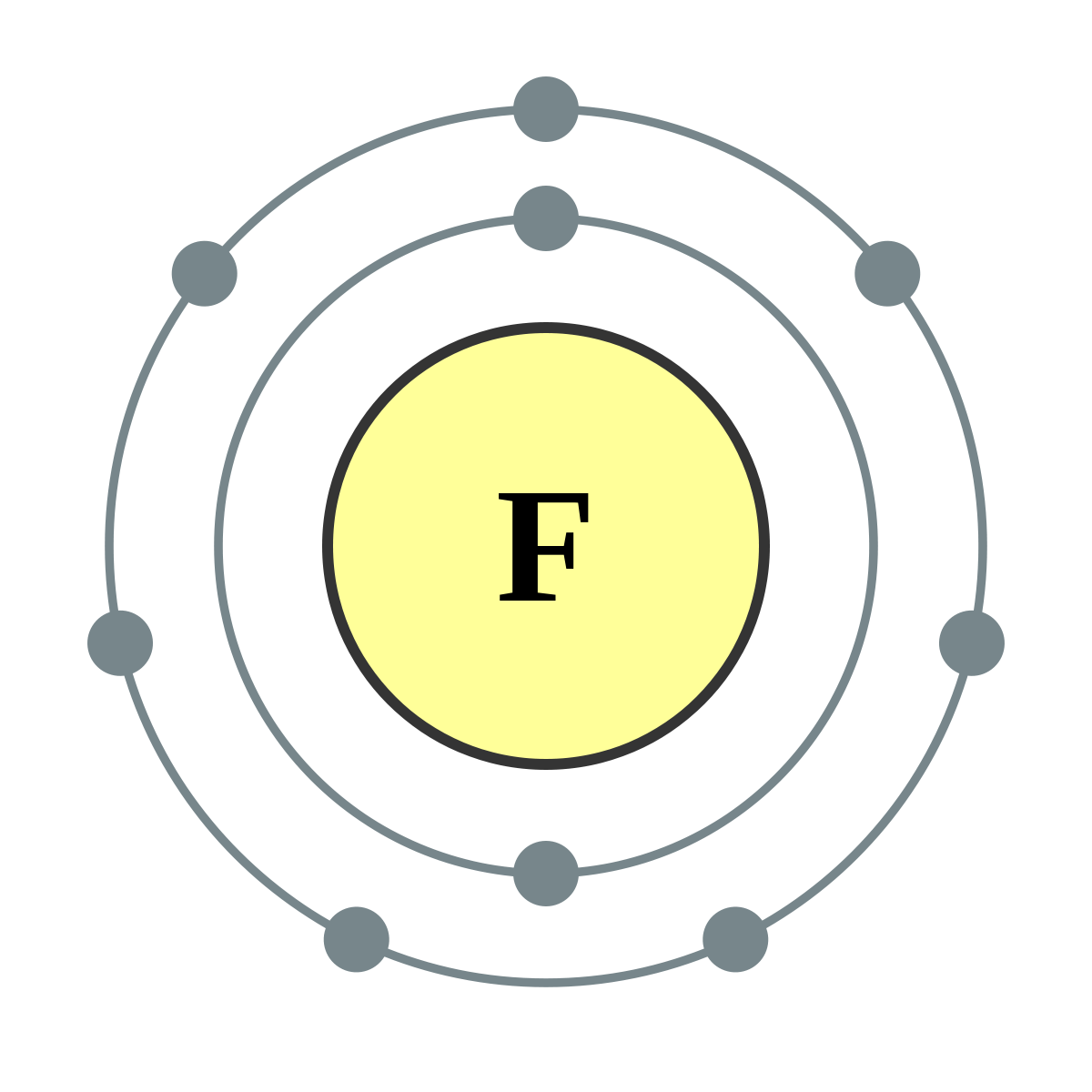
9
What is the name of the nuclear power plant that exploded in Russia in 1986?
Chernobyl
Which one is a physical change: ice melting, wood burning, or iron rusting?
Ice melting.
What family does calcium (Ca) belong to? Use your periodic table.
Alkaline earth metals
If an atom gains one electron, will its charge be positive or negative?
Negative (it becomes a negative ion)
In a Bohr model of lithium (Li), how many orbital levels are there?
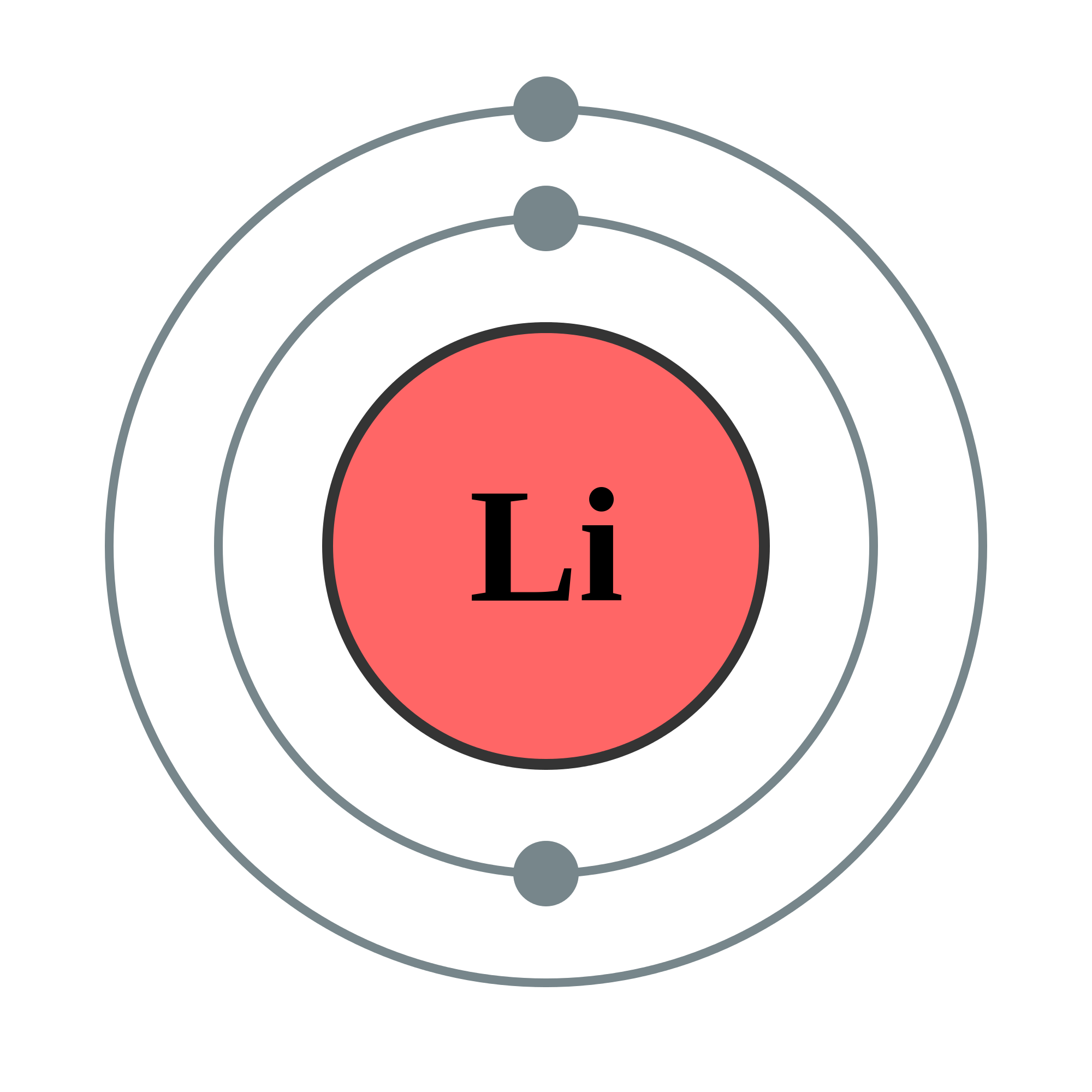
2
What do we call the nuclear process of splitting a heavy nucleus into two smaller ones, releasing energy?
Nuclear fission
Solid Iron is repeatedly smashed with a hammer in iron powder. Physical or Chemical change?
Physical
What family does fluorine (F) belong to? Use your periodic table.
Halogens
What is the difference between these two atoms?
Nitrogen-14 and Nitrogen-16
Any of the following: They are isotopes, different neutrons, different masses
Of the following choices, what two subatomic particles are found in the nucleus?
(Atom, proton, neutron, electron, mass, orbital, charge)

Protons and Neutrons
Which process happens in the extremely hot temperatures of the Sun: fission or fusion?
Fusion
In which state of matter are particles spread far apart and moving fast: solid, liquid, or gas?
Gas.
Elements like iron (Fe) and copper (Cu) are in which family? Use your periodic table.
Transition metals
How many neutrons does carbon-14 consist of?
Carbon-14 has 8 neutrons and 6 protons (8+6=14)
What parts of an atom contribute to its overall charge?

Protons and electrons
Which type of decay releases a helium nucleus containing 2 protons and 2 neutrons?
Alpha decay
Liquid Nitrogen is boiled at 800 degrees celcius (VERY HOT!) it evaporates into Nitrogen gas. Physical or chemical change?
Physical
Is silicon (Si) a metal, nonmetal, or metalloid?
Metalloid
An atom has 8 protons and 10 electrons. What is its charge?
-2 (It has gained 2 extra electrons)
What does the top number (the bigger number) represent in an isotope symbol like 146C?
The mass number (total protons + neutrons)
What is radioactivity?
The spontaneous breaking apart (decay) of unstable atomic nuclei.
Which state of matter has a fixed volume, but does NOT have a fixed shape. So it's volume doesn't change but its shape does. Solid, liquid, or gas?
Liquid