Solutions are ____________________ mixtures, uniform throughout.
What is homogenous?
An atom or molecule with a net electric charge due to the loss or gain of one or more electrons
What is an ion?
Chemical bond that involves the sharing of electron pairs between atoms.
What is covalent bond?
Type of reaction where a compound is created
(EX: 2H2 + O2 -> 2H2O)
What is synthesis?
Binary covalent compounds are between ___ different nonmetals.
What is "2"?
The part of a solution that is present in greater amounts.
What is a solvent?
A negatively charged ion
What is an anion?
Covalent bonds occur between 2 ________________.
What is non-metals?
Type of reaction where a compound is broken down
(EX: H2O2 -> H2 + O2)
What is decomposition?
Write the name of the first element as it appears on the periodic table, change the name of the second element to end with:
What is "ide"?
The part of a solution that is present in lesser amounts, dissolves in a solvent.
What is a solute?
A positively charged ion.
What is a cation?
The three types of covalent bonds.
What are single, double and triple?
Type of reaction where a compound plus oxygen combine to produce heat, CO2 and H2O.
What is combustion?
Prefix _______ means 1
Prefix _______ means 2
Prefix _______ means 3
What is mono, di, tri?
When two liquids mix:______________
When two liquids don't mix:______________
What is miscible/immiscible?
It is a type of chemical bond that generates two oppositely charged ions (complete transfer of valence electron(s) between atoms).
What is ionic bond?
______________ bonds are weakest
______________ are strongest
What is single, triple?
Type of reaction where one element replaces another in a reaction.
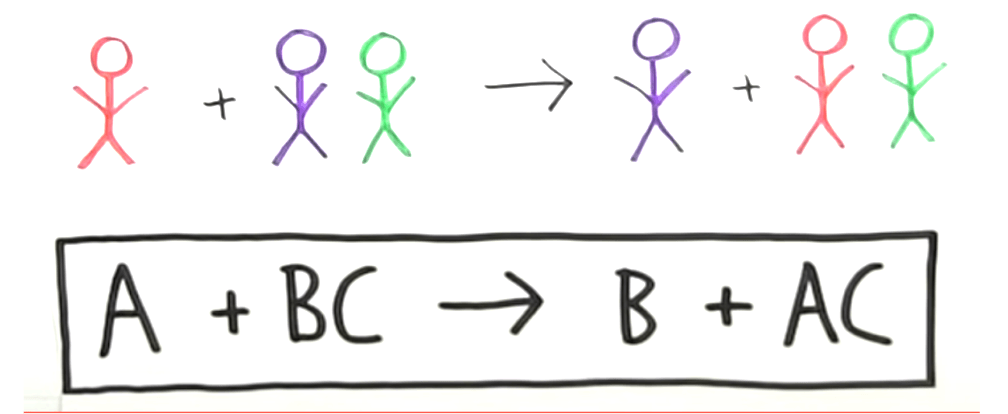
What is single replacement?
The prefix _______ is never used on the first element of a binary covalent compound. Without a prefix it is assumed that there is only 1.
What is mono?
When a mixture is NOT uniform all the way through, particles will settle/can be filtered out.
What is heterogeneous?
Ionic bonds form between a __________ and a ______________.
What is metal and non-metal?
The tendency of atoms to prefer to have eight electrons in the valence shell.
What is the octet rule?
The type of reaction where the positive and negative ions in two compounds switch places.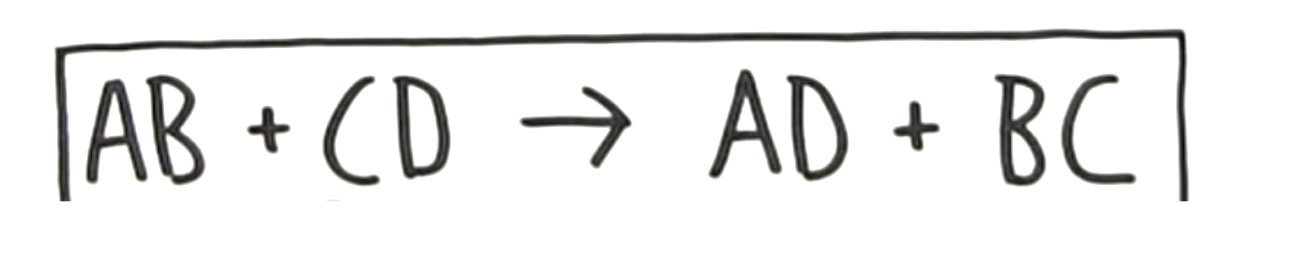
What is a double replacement reaction?
Name the following compound: N2O4
Dinitrogen tetroxide