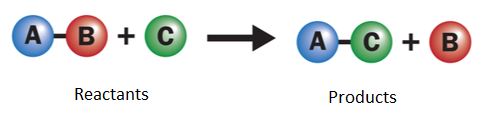
Balance the following chemical reaction:
3 Fe and 2 O
What is a mole?
A unit of measurement. Tells us how many things we have (molecules, atoms, particles, gummy bears, etc.)
What does a catalyst do?
Speeds up a reaction by lowering the activation energy.
What type of reaction is this?
Single replacement
Dry ice is an example of what type of phase change?
Sublimation
A+2B ⇌ C+D
If we take away some of B, which way does the system shift?
Left
How can we determine how many grams of an element are in one mole?
Periodic table atomic mass
What does the Law of Conservation of Mass say?
Atoms are neither created nor destroyed in a reaction. (Stuff doesn't just disappear)
This is what type of reaction?
Decomposition reaction
Name an element from the periodic table that has a high electronegativity.
Upper right
What does Le Chatelier's Principle say?
If a dynamic equilibrium is disturbed, the system will compensate for the change.
What is Avogadro's Number?
6.02 x 1023
In a chemical equation, what do we call the big numbers in front of the molecules? What do we can the small numbers within the molecules?
Coefficients, subscripts

This is what type of reaction?
Synthesis Reaction
We use distillation to separate what types of mixtures? Bonus (200): How does distillation work?
Liquids that have different boiling points. Boil the one with the lowest boiling point, it evaporates and condenses into separate container.
A(g) + 2B(g) ⇌ C(g) + D(g)
If we add pressure to the system, which way will it shift? And why?
Right
Place the following in order from least to greatest:
Atom
Mole
Electron
Molecule
Electron, Atom, Molecule, Mole
Balance the following equation:
P4O10 + H2O ---> H3PO4
1, 6, 4
Just by looking at a chemical equation, how can you tell if it is reversible or irreversible?
The single or double arrows
What is one way we can determine if one liquid is more dense than another?
pour into container and see which one is on top and bottom
What three things can cause a chemical equation to not have dynamic equilibrium (be out of balance)?
Concentration, temperature, and pressure
What is the difference between an element and a molecule?
Element: single substance found on periodic table
Molecule: many elements bonded together
Give an example of an exothermic reaction and an example of an endothermic reaction.
Exothermic: burning wood, yeast and hydrogen peroxide
Endothermic: photosynthesis, epsom salts in water
What type of reaction is this?
Double replacement
Draw a Bohr diagram of an atom of Magnesium.
Should have 11 electrons and 3 orbitals