What are the three States of Matter?
Solid, Liquid, and a gas
What are some example of physical change?
Evaporation, Ice cube melting, Water freezing, crushing a piece of paper, etc.
What are some example of Chemical Change?
Rust on a car, the statue of Liberty, rotten food, a battery corrosion, etc.
What is a pure substance that cannot be separated into simpler substances?
Element
When separating mixtures what is a filtration process during which a solid changes to a vapor without melting. An example is dry ice.
Sublimation
What is it called when Atoms with the same numbers of protons, but has different numbers of neutrons?
Isotopes
What is the equation for finding the Mass Number
Mass Number = Atomic Number + Number of Neutrons
True or False
If unstable nuclei lose energy by emitting radiation in a spontaneous process, is it called radioactive decay?
True
What is the smallest particle of matter that retains the properties of the element?
1) Compound
2) Atom
3) Electron
4) Micromatter
Atom
True or False
Are Gamma rays positive and they do reflect by electric or magnetic field?
False
Common sense
On the electromagnetic spectrum what has the most Energy/frequency Increases?
Gamma Rays
In this configuration [Ne]3s^1, why do we use the brackets?
The [Ne] is a noble gas and we use it because it shortens the electron configuration.
What is the shape of a p orbital?
Dumbbell-shaped
What is the element from this electron configuration?
1s^2 2s^2 2p^6 3s^2 3p^4
Sulfur(s)
What is electromagnetic radiation?
A form of energy that exhibits wavelike behavior as it travels through space.
In which group has one electron in their outermost energy level?
Group 1
In this electron configuration, what is the group and period that this element is in, and what element is it.
1s^2 2s^2 2p^6 3s^2 3p^2
Group 14, Period 3
Element is Silicon (Si)
Elements in the same group have the same what?
Have the same number of valence electrons.
True or False
The f block has 15 columns on the periodic table.
False
The f block has 14 columns because the elements Lanthanum (La) and Actinium (Ac) are in d block.
What are the elements classified as?
The 3 are: Metals, Nonmetals, and metalloids.
NOT Synthetic because they are actual chemical elements not natural elements.
What is the difference between Baking soda and Gold. Also are they a chemical or physical change.
Baking soda is a compound and Gold is an element and they are a chemical change.
Who's Experiment is this?
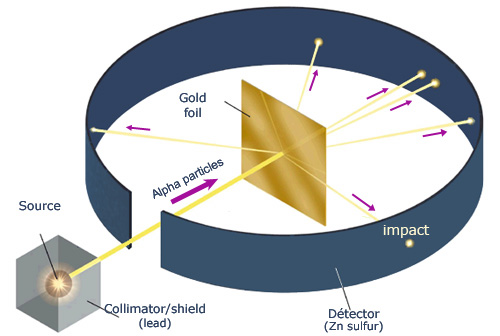
Rutherford's Experiment.
For light waves, you can use the formula c = λv to calculate the wavelength or frequency of any wave. What does the c, λ, and v represent?
C = is the speed of light in a vacuum
λ = is the wavelength
v = is the frequency
What 2 groups in the periodic table are the most reactive?
Group 1 because they are alkali metals. They only exist as compounds with other elements.
Group 17 because they are halogens. They are part of compounds.
What is the percent by mass formula.
