If there is a spill or other emergency in the lab, this is the first thing we do.
What is tell the teacher?
Solubility (Dissolving)
What is a physical property?
Water
What is a compound?
A test tube is knocked over and shatters.
What is a physical change?
Mass
What is an extensive property?
A mixture that is uniform throughout.
What is a homogeneous mixture?
The physical property this object measures: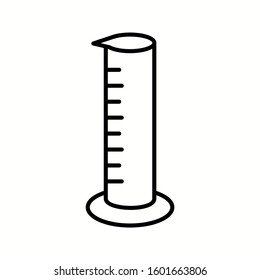
What is volume?
Reactivity
What is a chemical property?
Table Salt
What is a compound?
Water is frozen into ice.
What is a physical change?
Color
What is an intensive property?
Matter with a definite shape.
What is a solid?
The best tool for protecting your eyes in a lab.
What are goggles or safety glasses?
Flammability (Burns)
What is a chemical property?
Oxygen
What is an element?
Paper combusts (catches on fire).
What is a chemical change?
Boiling point
What is an intensive property?
A mixture that is not uniform throughout.
What is a heterogeneous mixture?
An object that can be used to extinguish flames on a person's clothing.
What is a fire blanket?
Color
What is a physical property?
Sugar
What is a compound?
Iron rusts.
What is a chemical change?
What is an extensive property?
Matter with no definite volume.
What is a gas?
The proper place to dispose of broken glass.
What is the broken glass container?
Luster
What is a physical property?
Lithium
What is an element?
Gold dissolves in acid.
What is a physical change?
Volume
What is an extensive property?
An example of something that is not matter.
What is light?