What group is column 8?
Noble Gases
Cations are ___________ charged ions.
Positively
Isotopes have the same ____ but different ___.
atomic number (number of protons), mass number (number of protons + neutrons)
What is atomic mass?
The atomic mass for each element appearing on the periodic table represents the weighted average of masses for each individual isotope of an element
What the difference between an element, ion and isotope?
Element- different number of protons
Ion- different number of electrons
Isotope- different number of neutrons
What is the oxidation charge of group 5A?
-3
Elements in group 2A for ions with this charge.
2+
Complete the following:
Isotope name: Oxygen-17
atomic#___ mass#____ #of protons ____ #of neutrons_____ #of electrons_____
Atomic #: 8
Mass #: 17
# of Protons: 8
# of Neutrons: 9
# of Electrons: 8
An element with 5 protons, 6 neutrons, and 8 electrons has a mass number of ___.
11
Which about isotopes is true?
a. they have the same number of neutrons
b. they have a different atomic number
c. they have the same number of protons
C. They have the same number of protons
What are the 3 parts of an atom?
Protons, electrons, and neutrons
Anions are formed by (metal or nonmetal)
Nonmetal
Arsenic isotope is written as As-85 with 33 protons, how many neutrons does it have?
52 neutrons
The mass of the most common isotope of an element is known based on an element's ____.
Average atomic mass.
How do you calculate the average atomic mass of an isotope?
Multiply the mass of each isotope by its percent abundance then divide by 100
A.) This element is in the same family as Br, but has fewer protons than Mg. It must be ____.
B.) This element has 6 energy levels and 8 valence electrons. It must be ____.
A.) F (Fluorine)
B.) Rn (Radon)
Cations are __________ om size than the atoms from which they are formed.
smaller
Why are these two atoms not isotopes?
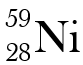
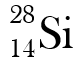
They have different atomic numbers.
If Boron-10 with an abundance of 19.78% 10.013 amu and Boron-11 with an abundance of 80.22% 11.009 what is the average atomic mass?
10.81 amu
How many elements have been discovered?
118
A.) This element is in the alkaline earth metals group and has 3 energy levels. This must be____.
B.) This element is in the family with the most reactive non metals and has 4 energy levels. It must be____.
A.) Mg (Magnesium)
B.) Br (Boron)
Explain the difference between a cation and an anion and how can you determine the change of an ion based on its position on the periodic table?
A cation is a positive and anion is negative. Metals tend to form cations and non metals form anions.
Solve the following:
Uranium-238
Atomic #: 92
Mass #: 238
# of Protons: 92
# of Neutrons: 146
# of Electrons: 92
If Sulfer-32 93.12% 31.928 amu and Sulfer-34 6.88% 34.023 what will the average atomic mass?
32.07 amu
What is the number of protons, neutrons and electrons in Tin-120?
Protons=50
Neutrons=70
Electrons=50