By definition, what makes nitrogen (N) and oxygen (O) different elements?
Nitrogen atoms each have 7 protons, while oxygen atoms each have 8.
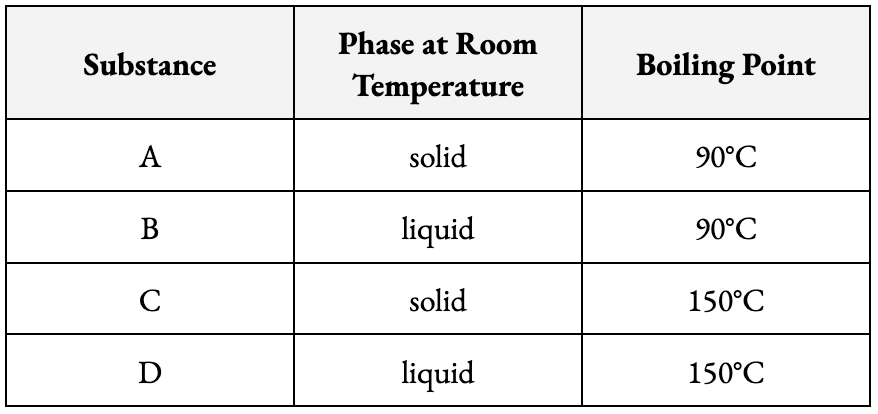
Which two substances above would you separate from each other using distillation?
B and D
What is the difference between an intermolecular and intramolecular forces?
Intermolecular forces occurs between molecules. Intramolecular forces occurs within a molecule
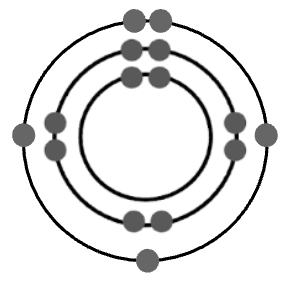
Which element is represented by the Bohr diagram above?
Phosphorus
The following track title is from Sufjan Stevens's album "Illinois". Without writing, memorize the title and repeat it back the next time this category is chosen. [Level 1]
The Predatory Wasp of the Palisades is Out to Get Us!
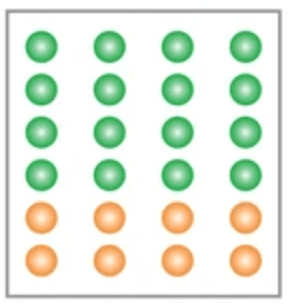 What type of substance is represented by the image above?
What type of substance is represented by the image above?
Heterogenous mixture
A sample of mixed gasses contains 25.0% argon gas and 75.0% nitrogen gas. The total pressure of the two gasses combined is 60.0 Pa. What is the partial pressure of the nitrogen gas?
45.0 Pa
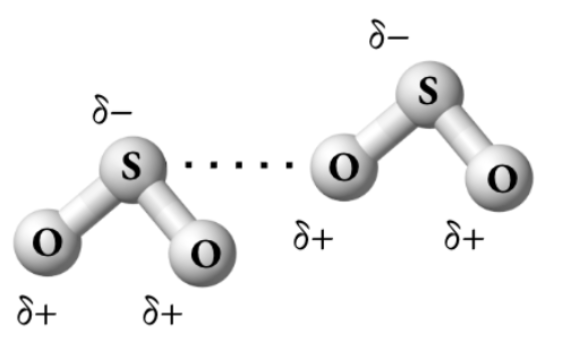
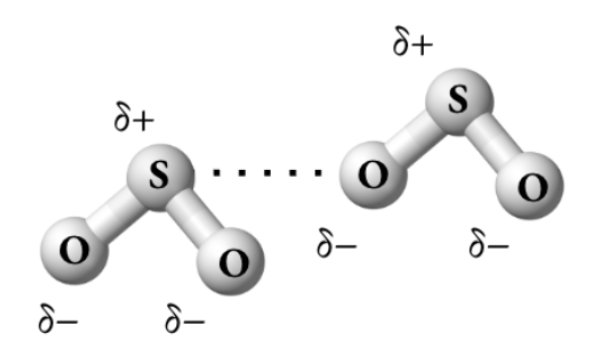
The diagram above shows dipole-dipole forces between oxygen (electronegativity = 3.44) and sulfur (electronegativity = 2.58).
Correct a mistake in the diagram.
Oxygen should get the partial negative charge (and sulfur the partial positive charge) because oxygen has a higher electronegativity than sulfur. Below is a corrected diagram:
Elements with the same number of _____ tend to have similar properties, such as reactivity.
Valence electrons
The following track title is from Sufjan Stevens's album "Illinois". Without writing, memorize the title and repeat it back the next time this category is chosen. [Level 2]
They are Night Zombies!! They Are Neighbors!! They Have Come Back from the Dead!! Ahhh!
Glucose, a molecule, has the formula C6H12O6. If a sample of glucose contains 18 trillion carbon (C) atoms, how many hydrogen (H) atoms does the sample contain?
36 trillion
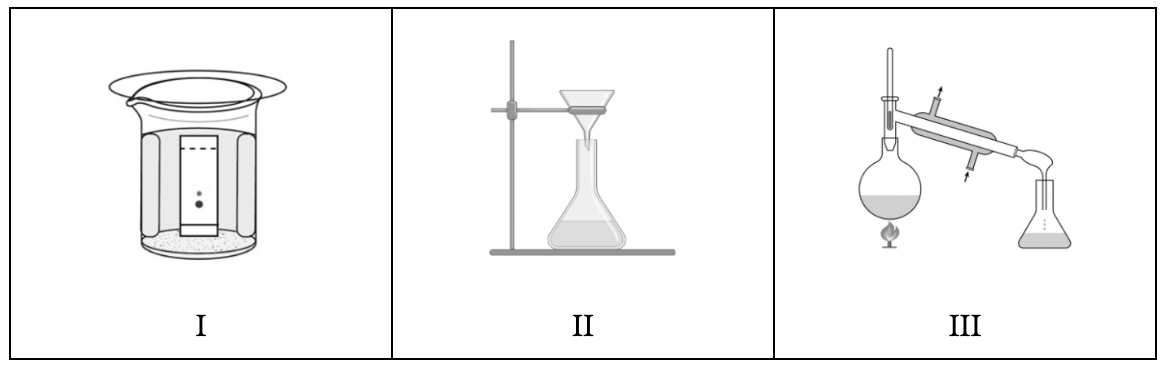
Which mixture-separation method is represented by each of the diagrams above?
I = chromatography
II = filtration
III = distillation
List 3 properties affected by intermolecular forces
Melting point, boiling point, volatility, surface tension, solubility, etc.
Which 2nd-period element has 4 valence electrons?
Carbon
The following track title is from Sufjan Stevens's album "Illinois". Without writing, memorize the title and repeat it back the next time this category is chosen. [Level 3]
A Conjunction of Drones Simulating the Way in Which Sufjan Stevens has an Existential Crisis In the Great Godfrey Maze
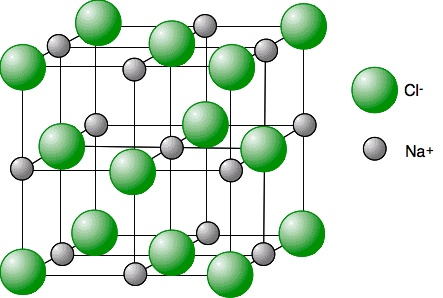
Above is a diagram of sodium chloride (NaCl), composed of sodium (a metal) and chlorine (a nonmetal). Is sodium chloride a compound, molecule, or both?
Compound
In a mixture of nitrogen and oxygen gasses, the partial pressure of the nitrogen gas is 0.5 atm and the partial pressure of the oxygen gas is 0.75 atm. If the mixture contains 4.5 × 1023 oxygen atoms, how many nitrogen atoms are in the sample?
3.0 × 1023 oxygen atoms
What is the difference between how London dispersion and dipole-dipole forces are formed? Which is stronger?
London dispersion forces are caused by random electron movement, whereas dipole-dipole forces are caused by differences in electronegativity. Dipole-dipole forces are stronger and more permanent than London dispersion.
What is electronegativity? Which element has the highest electronegativity?
Electronegativity is a measure of how strongly an atom attracts shared electrons in a chemical bond. Electronegativity increases from the bottom left to the top right of the periodic table (excluding the noble gasses, which don't bond): Fluorine has the highest electronegativity.
The following track title is from Sufjan Stevens's album "Illinois". Without writing, memorize the title and repeat it back the next time this category is chosen. [Level 4]
Riffs and Variations On a Single Note for Jelly Roll, Earl Hines, Louie Armstrong, Baby Dodds, and the King of Swing, to Name a Few
What is the difference between a pure substance and a mixture?
The components of pure substances exist in fixed ratios, unlike in mixtures.
How does chromatography work to separate a mixture?
As the mixture travels through another substance, each component of the mixture travels at a different speed, thus separating the mixture.
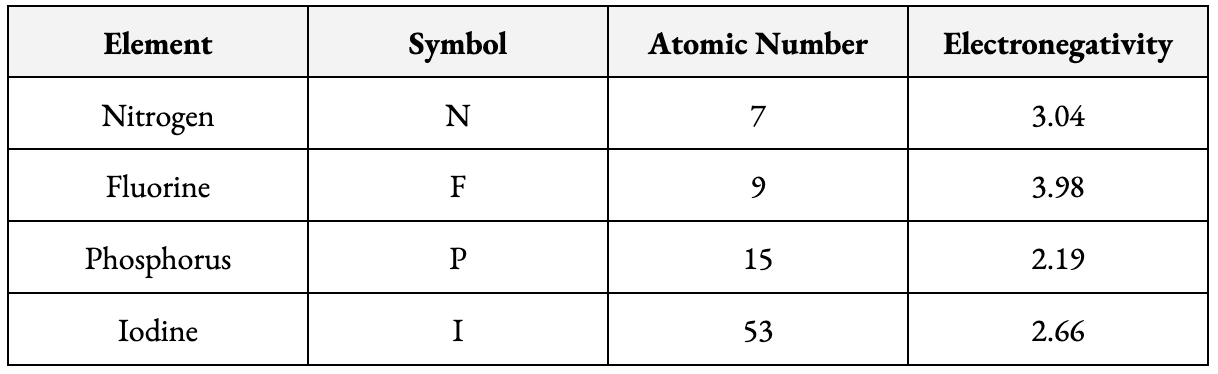
Based on the table above, which substance likely has a higher boiling point: nitrogen trifluoride (NF3) or phosphorus triiodide (PI3)? Why?
NF3, because it is more polar than PI3. Higher polarity means stronger dipole-dipole forces, which means it takes more energy to overcome the intermolecular forces and boil.
NF3 is more polar than PI3 because the difference in electronegativity between F and N (0.94) is greater than the difference in electronegativity between I and P (0.47).
Why does the atomic radius get smaller as we go from left to right across a period?
As the number of protons and electrons increases, the negatively-charged electrons feel a stronger pull toward the positively-charged nucleus, slightly decreasing the atom's size.
The following track title is from Sufjan Stevens's album "Illinois". Without writing, memorize the title and repeat it back the next time this category is chosen. [Level 5]
The Black Hawk War, or, How to Demolish an Entire Civilization and Still Feel Good About Yourself in the Morning, or, We Apologize for the Inconvenience but You're Going to Have to Leave Now, or, 'I Have Fought the Big Knives and Will Continue to Fight Them Until They Are Off Our Lands!'