What is an Isotope?
Isotopes are atoms of the same elements with the same number of protons but different numbers of neutrons and mass numbers.
What atomic theory did John Dalton create?
John Dalton is known as the founder of the Atomic Theory. He created the solid sphere model.

How do you draw the Lewis Dot Diagram?
1) Write the element symbol
2) Draw the valence electrons in a circle around the symbol
What is the longhand electron configuration for Hydrogen?
1s1
What charge does Group 1 have?
+/1+
What is an Ion?
An ion is an atom or molecule with a charge due to the loss or gain of electrons. There are 2 types of ions:
Cations: Positively charged ions due to the loss of electrons
Anions: Negatively charged ions formed by gaining electrons
What is the Plum Pudding Model of the atom?
The Plum Pudding Model was created by Josephy John Thomsom (J.J. Thomson). He used cathode rays (electron beams) to discover the electron. Electrons are small, negatively charged subatomic particles.
How do you draw the Bohr Model?
1) Write the element symbol.
2) Draw the number of shells in the element around the symbol.
3) Fill in the number of electrons in each shell.
What is the Longhand and Shorthand electron configuration of Carbon?

Longhand: 1s22s22p2
Shorthand: [He]2s22p2
What charge does Group 4A have?
4+
What is Ionization Energy?
The Energy required to remove an electron from an ion. Ionization Energy Increases across a period and up a group.
What is the Nuclear Model?
Ernest Rutherford created the Nuclear Model of the atom. He discovered that the atom is mostly empty space and that electrons are located outside the nucleus. The Nucleus is a dense, positive core in the center of the atom.
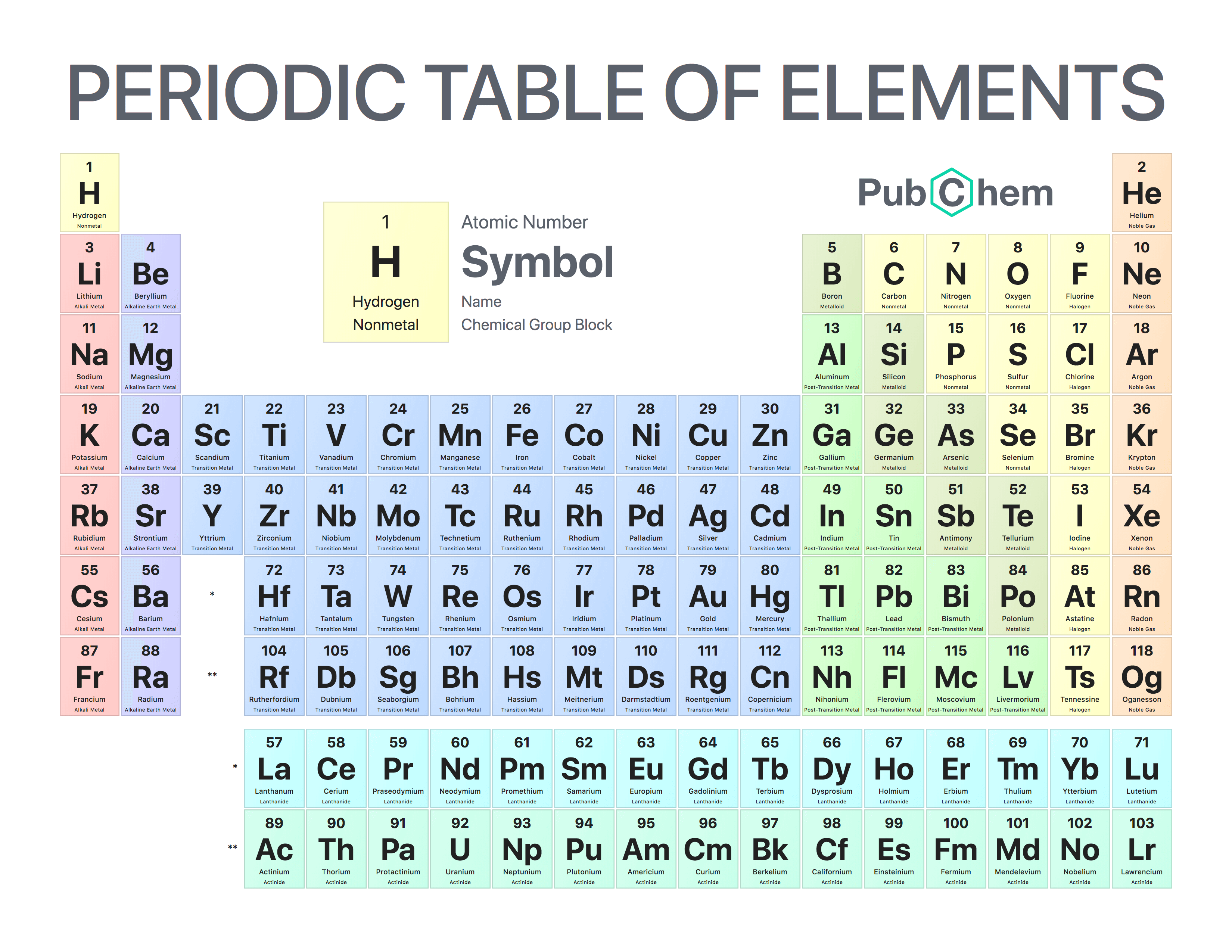
How was the periodic table organized?
Increasing atomic number
Write the shorthand electron configuration of Barium

[Xe]6s2
What charge does Group 6A have?
2-
What is Electronegativity?
Electronegativity is the ability of an atom to attract electrons in a chemical bond. Fluorine has the highest electronegativity.
What Atomic Model did Neils Bohr develop?
Neils Bohr created the Bohr/ Planetary Model of the atom. He discovered that electrons travel around the nucleus in orbitals.
What is The Octet Rule?
Gaining or losing electrons to achieve 8 valence electrons. The Octet Rule states that the 1st shell in an atom can hold up to 2 electrons. The shells after the first can hold up to 8.
What is the longhand and shorthand electron configuration of Silicon?

Longhand: 1s22s22p63s23p4
Shorthand: [Ne]3s23p4
What charge does Group 7A have?
-/1-
What is the periodic table?
The periodic Table was developed by Dimitri Mendeleev in 1863. It displays elements by atomic number and similar chemical properties of the elements. There are 7 periods and 18 groups.
What atomic model did Erwin Schrodinger develop?
Erwin Schrodinger created the Quantum/Electron Cloud/Wave Mechanical model of the atom. He discovered that electrons exist in areas of high probability of finding an electron. He also discovered the uncertainty principle (you cannot know both the speed and position of an electron at the same time).
What is electron affinity?
The energy change when an atom gains electrons.
Write the longhand and shorthand electron configuration of Pottasium.

Longhand: 1s22s22p63s23p64s1
Shorthand: [Ar]4s1
What are the 9 polyatomic ions?
Sulfate (SO4), Ammonium (NH4), Nitrate (NO3), Carbonate (CO3), Phosphate (PO4), Acetate (C2H3O2), Hydroxide (OH), Cyanide (CN), Hydrogen Carbonate (HCO3)