The electron configuration of Helium, which has the atomic number "2"
What is 1s2? or, What is "Two electrons in the first shell"?
The center of the atom, where you find protons and neutrons.
What is the nucleus?
These electrons are located in the outermost shell of an atom's electron energy levels. They love to share!
What are Valence Electrons?
The solid sphere model doesn't show these two things as separate, unlike the nuclear model.
What are the nucleus and electrons?
The more energy a photon has, the ______ its wavelength is.
What is "lower" or "shorter"?
The element with electron configuration 1s22s22p5
What is Fluorine (F)?
The positively-charged part of a nucleus.
What is a Proton?
The name for a particle of light, emitted when electrons lower their energy level
What is a Photon?
This model, also called the Bohr model, shows electron energies as "orbits".
What is the Planetary model?
Light can be dangerous! This kind of light can give us sunburn, skin cancer, and a way to clean our safety glasses here in class.
What is "ultraviolet" or UV?
The electron configuration of Sodium, which has the atomic number "11"
What is 1s22s22p63s1?
The negatively-charged particles surrounding the nucleus.
What are Electrons?
The process by which atoms release photons; happens when electrons fall down from high energy to low energy.
What is emission?
This model is the most classic visual of an atom, but doesn't show energy levels of electrons. You can see it represented here, in the Isotopes logo!
What is the Nuclear Model?
This tool, which we used to see emission lines, is just a tube with some diffraction lenses in it.
What is a spectroscope/spectroscope tube?
The element with electron configuration 1s22s22p63s23p3
What is Phosphorus (P)?
The number of neutrons that Oxygen-18 must have
What is 10?
This is how we find the atomic mass, or the total number of particles in the nucleus
What is "add protons and neutrons together"?
This model shows electrons swimming around in the atom. Still doesn't show a nucleus, though!
What is "Plum pudding"?
This spectrum shows four lines in what kind of light? Hint: it's the kind we can see!
What are "Visible" or "Optical" wavelengths?
The electron configuration of Iron, which has the atomic number "26"
What is 1s22s22p63s23p64s23d6?
What are Isotopes?
This is the term for a bonded set of atoms. Oh no? More like O2!
What is "Molecule"?
The quantum model of atoms shows electrons not as orbits, but as these, which could be a sign of rain if you see them in the sky.
What are clouds?
Which two elements make up our "unknown mixture"?
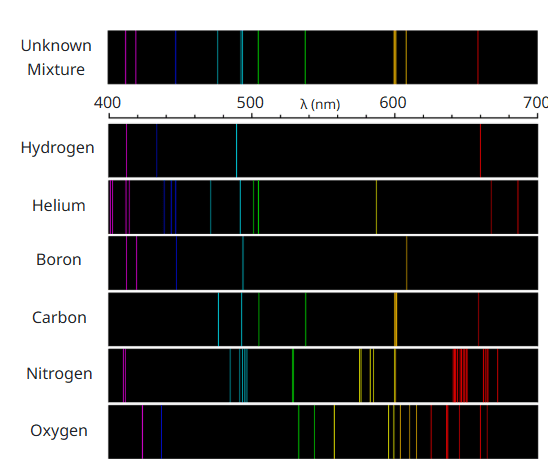
What is "boron" and "carbon"?