Define Matter.
What is Matter entirely made out of?
Matter is anything that has a mass and takes up space.
Matter is mate entirely of atoms!
Draw the EELD Diagram for:
Potassium & Chlorine
Draw the Bohr Model for Oxygen & Potassium
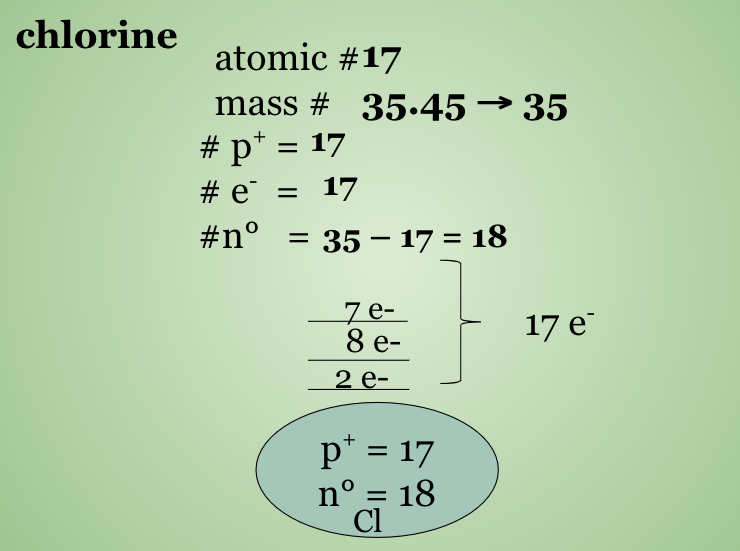
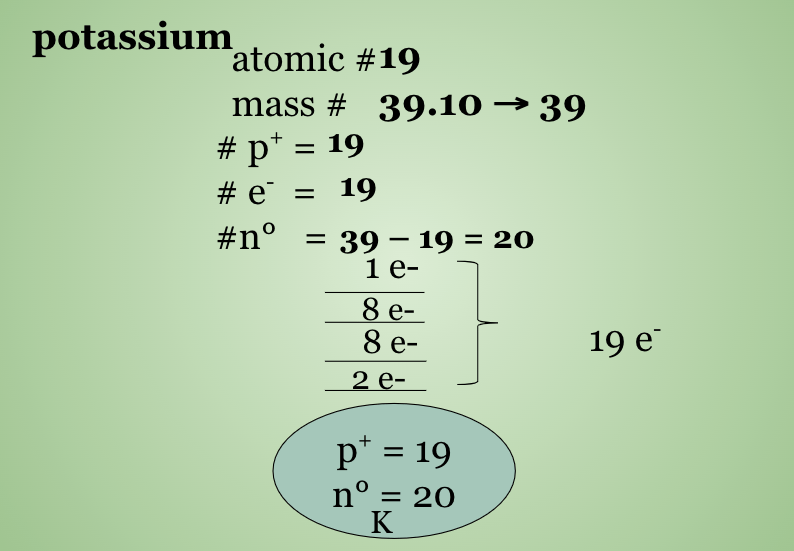
Oxygen & Potassium Bohr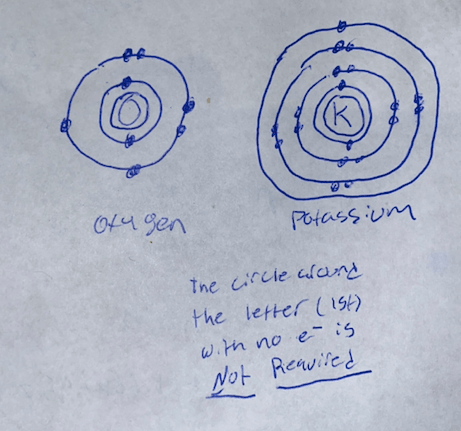
PLEASE REMEMBER THE FIRST TWO ELECTRONS ARE DRAW BESIDE EACH OTHER, THESE DRAWINGS HAVE THEM 180º APART WHICH ISW WRONG!
What is an Ion? What is an Isotope?
Ions of Sodium bond to Ions of Chloride using what type of bonds?
Ions of Oxygen bond to Ions of Nitrogen using what type of bonds?
Ions:
Ions are atoms or groups of atoms that have a net charge --> the number of p+ and e− are not equal
Isotopes:atoms that have the same number of protons but a different number of neutrons
Ionic Bonds
Covalent Bonds
Name the following compounds: (for acids, IUPAC & Classical Names)
H2SO4(aq) & CO3
1. C = Sulfuric Acid IUPAC = Aqueous Hydrogen Sulfate
2. carbon trioxide
Name four properties of an Acid & four properties of a Base
(if you use it litmus is one property)
Acids:
always soluble in H2O
conducts electricity
neutralize bases
taste sour
reacts with metals to produce H2(g)
Aqueous
When they are dissolved in water, we say they “ionize"
Bases:
usually soluble in H2O
conducts electricity
neutralize acids
taste bitter
feel slippery
Are usually ionic hydroxides (ie. contain OH- ion).
When they are placed in water we say they “dissociate”
In chemistry, a mole refers to:
one mole refers to: 6.02 x 1023 atoms, molecules etc!
Which artist has the most grammy awards?
The Queen --> Beyoncé herself:

Who is the father of the Periodic Table?
What is the pattern that he noticed called? (Explain)
Groups 1, 2, 3-12, 17 & 18 are called?
Dmitri Mendeleev & the pattern he noticed is the Periodicity of elements: the repeating pattern in physical & chemical properties of the elements on the periodic table
1. Alkali Metals 2. Alkaline-Earth Metals
3-12. Transition Metals 17. Halogens
18. Noble Gases
Liquid Water decomposes into it's elements.
--> write the balanced Chemical Equation with states
2 H2O (l) --> 2 H2 (g) + O2 (g)
What is the Atomic Structure of the Isotope Bismuth - 224
Atomic # = 83
Isotope atomic mass = 224 g/mol
# protons & electrons = 83
#neutrons = 224 - 83 --> 141 neutrons
Provide the formula the following compounds (include states):
lead (II) nitrate & Sulfur
1. Pb(NO3)2(aq)
2. S8(s)
Name the following compounds: (For acids, IUPAC & Classical names)
NaOH (aq) & HNO3 (aq)
1. Sodium Hydroxide (aq)
2. Nitric Acid (aq) or Aqueous Hydrogen Nitrate
What are the two formulas for the Mole?
provide units where apporiate
1. n = m / M
n = moles in mol
m = mass in g
M = molar mass in g/mol
2. n = # of atoms/Avogadro's #
n = moles in mol
Avogadro's # = 6.02 x 1023
Name three of the 5 highest grossing movies of all time:
1. Avatar --> $2,923,706,026
2. Avengers: Endgame --> $2,797,501,328
3. Avatar: The Way of Water --> $2,320,250,281
4. Titanic --> $2,257,844,554
5. Star Wars: The Force Awakens --> $2,068,223,624
What subatomic particles are located within:
1. in the nucleus
2. in the cloud region (within energy levels)
Also what charges do each type of subatomic particle have?
On the periodic table -
How do you find the number of:
Electrons, Protons and Neutrons
Give me an Example!
1. Neutrons (Neutral) & Protons (Positive)
2. Electrons (Negative)
Electrons = The Atomic Number
Protons = The Atomic Number
Neutrons = The Atomic Mass - The number of Protons
Aluminium:
Electrons = 13
Protons = 13
Neutrons = 26.98-13 --> 13.98 --> 14
(NH4)3PO4 ( ) + Ba(OH)2 ( ) --> X ( ) + Y ( )
Predict the products & the states!
(NH4)3PO4 (aq) + Ba(OH)2 (aq) --> NH4OH (aq) + Ba3(PO4)2 (s)
Why do Cations give up their extra electrons, or why do Anions accept extra electrons when they are in their ionic state?
Because they are trying to fill/empty their valence shell. Elements with complete valence shells (the noble gases) are extremely stable, ions donate/accept electrons to try and achieve that stable configuration!
Name the following compounds:
C12H22O11 & Na2SO4
1. sucrose
2. sodium sulfate
Explain the Naming Rules for Acids & Bases
Acids
1. Name compound as an ionic compound
- Hydrogen ______ide --> Hydro___ic Acid
- Hydrogen ______ate --> _______ic Acid
- Hydrogen ______ite --> ______ous Acid
*STATE IS ALWAYS aq
Bases: same rules as ionic compounds because most bases are ionic except ammonia - NH3
1. The Cation stays the same
2. OH = Hydroxide
NaOH = Sodium Hydroxide
How many molecules are present in 0.75 mol of H2O?
# atoms = n x Avogadro's #
0.75 mol x 6.02 x 1023 = 4.515 x 1023 -->
~4.5 x 1023 molecules are present in 0.75 mol of H2O
Walk on the living, they don't even mumble. Walk on the dead, they mutter and grumble. What are they?
Leaves!
What do Elements in the same group (ignoring the 1 in groups 13-18) have in common?
All non-metallic ions:
a. have fewer electrons than protons
b. have more electrons than protons
c. have more protons than electrons
d. have less protons than neutrons
They have the same number of valence electrons!
b. more electrons than protons! Non-metal ions have a net negative charge, which means they have more electrons than protons!
CH4 burns in air to provide fuel for a natural gas barbecue
CH4(g) + 2 O2(g) --> CO2(g) + 2 H2O(g)
Air = distractor
Fuel = CO2 --> Hydrocarbon combustions always produce CO2 (g) + H2O (g)
Name the Ion for the following elements:
Na, Ir, Ge & F
What are the Diatomic & Polyatomic Molecules (Formulas!)
--> Bonus 100 points if you can tell me when they are in their diatomic, polyatomic state
Na2+ = Sodium Ion
Ir4+ = Iridium Ion
Ge4+ = Germanium Ion
F- = Fluoride Ion
H2, N2, F2, O2, I2, Cl2, Br2, P4, S8
Bonus - They are in this state when they are alone!
Example: CH4 does not follow the rule of H2, because Hydrogen is not on its own (it's bonded to Carbon)
"Produces hydrogen gas" follows the rule of H2 (g) because Hydrogen is on it's own (not bonded to anything)
Sulfur reacts with Oxygen to produce Sulfur Oxide Gas
(Convert the chemical equation to a word equation - including states) - What type of Reaction is it?
2 LiOH (s) + H2SO4 (aq) --> Li2SO4 (aq) + 2 H2O (l)
S8 (s) + 4 O2 (g) --> 8 SO (g)
Lithium hydroxide (solid) reacts with sulfuric acid (aqueous) to form lithium sulfate (aqueous) and water (liquid).
Name the following compounds in (for acids, both IUPAC & Classical)
- H2SO3(aq)
- H2CrO4(aq)
Name the following compounds:
- Ca(OH)2(s) & NH3
IUPAC: aqueous hydrogen sulfite
Classical: sulfurous acid
IUPAC: aqueous hydrogen chromate
Classical: chromic acid
- Calcium Hydroxide & Ammonia
How many moles are in 0.25 g of Al(NO3)3
Show all of you work & put your answer into scientific notation
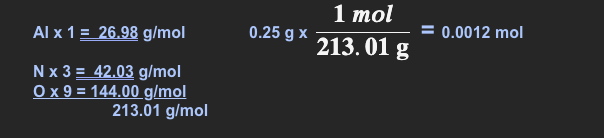
~ 1.2 x 10 -3 mol (scientific notation)
Finish the lyric: (100 bonus points if you sing it!)
Just a city boy, born and raised in South Detroit
__ ______ ___ _______________ _____ _____ ________
Just a city boy, born and raised in South Detroit
He took the midnight train going anywhere!
Where do electrons exist? What happens to an electrons position when they gain or lose energy?
1. Alkali Metals 2. Halogens 3. Alkaline Earths
4. Metalloids 5. Transition Metals 6. Noble Gases
Classify the elements below with the names in the list above
rubidium is an example of a(an)____________
strontium is an example of a(an)____________ xenon is an example of a(an)____________
iodine is an example of a(an)____________
Electrons orbit the nucleus at specific distances, inside specific energy levels!
- Electrons that absorb (gain) energy can be be temporarily excited to a higher energy level, and they emit (lose) energy as they return to their original level.
Rb = Alkali Metal (Group 1)
Sr = Alkaline Earth Metals (Groups 2)
Xe = Noble Gas (Group 18)
I = Halogen (not a gas though!)
Why is it necessary for us to Balance chemical equations? (looking for a specific concept in the answer)
Because the Law of Conservation of Matter states that matter cannot be created or destroyed, only transferred or transformed. This means in a chemical reaction the number of element s & the total mass of reactants and products needs to be equal!
Name or provide the formula for the following Ionic compounds:
1. YPO4
2. Strontium Borate
3. (NH4)2O
Name or provide the formula for the following compounds:
1. carbon tetrachloride
2. P7O10
3. diboron pentabromide
4. C2N3
1. Yttrium Phosphate
2. Sr3(BO3)2
3. Ammonium Oxide
1. CCl4
2. Heptaphosphorus Decoxide
3. B2Br5
4. Dicarbon Trinitride
Balance & Identitfy type of Reaction
1. __ NaBr + __ Ca(OH)2 --> ___ CaBr2 + ____ NaOH
2. ____P4 + ____O2 --> ____P2O3
3. ____ C7H16 + ____ O2 --> ____ CO2 + ____ H2O
4. ___Pb + __ H3PO4 --> ____ H2 + ____ Pb3(PO4)2
1. 2 NaBr + ____ Ca(OH)2 --> CaBr2 + 2 NaOH (Double Replacement RXN)
2. ___P4 + 3 O2 --> 2 P2O3 )- (Formation RXN)
3. _____C7H16 + 11 O2 --> 7 CO2 + 8 H2O (Combustion RXN)
4. 3 Pb + 2 H3PO4 --> 3 H2 + ____ Pb3(PO4)2 (Single Replacement RXN)
Write the formula for the following compounds (include states)
1. aqueous hydrogen nitrite
2. acetic acid
3. hydrophosphoric acid
4. hydrogen
5. aqueous hydrogen cyanide
1. HNO2(aq)
2. CH3COOH (aq)
3. H3P (aq)
4. H2 (g)
5. HCN (aq)
What mass (in grams) must be weighed out to get exactly 4.2 moles of C2H5OH? Show all work & put answer into scientific notation!
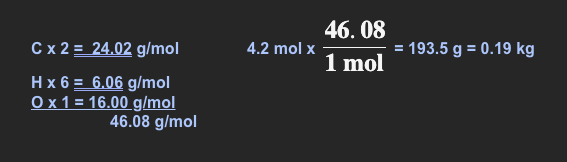
- ~1.9 x 102 g (Scientific notation)
How many Letters are in the Latin Alphabet? (Closest guess if the first group isn't close)
21 No G, J, U, W, Y
