The positive particle in an atom
What is a proton?
The ion formed when an atom gains an electron
What is an anion or negative ion?
the law stating that the mass of the reactants = the mass of the products
What is the Law of Conservation of Mass?
The reaction H+ + OH- —> H2O
What is neutralisation?
The name of the group 1 elements
What are the alkali metals?
The number of protons in an atom’s nucleus is known as this.
what is the atomic number
The formula of aluminium oxide
What is Al2O3 ?
The number of hydrogen atoms on each side of this balanced equation: 2H2 + O2 —> 2H2O
What is four?
The products of the reaction of sodium hydroxide and sulfuric acid
What are sodium sulfate and water?
The name of the group of unreactive elements.
What are the noble gases?
Carbon -12 and carbon-14 are examples of
What are isotopes
The formula of magnesium hydroxide.
What is Mg(OH)2 ?
The coefficients needed to balance this question.
CH4 + O2 —> CO2 + H2O
1: 2 : 1 : 2
The products of the reaction when zinc carbonate is heated.
What are zinc oxide and carbon dioxide?
The elements like silicon that have properties of both metals and non-metals
What are the semi-metals or metalloids?
The scientist who proposed that most of an atom is empty space.
Who is Rutherford?
The chemical formula for dinitrogen tetroxide
What is N2O4 ?
The coefficients needed to balance this equation
Zn + HCl —> ZnCl2 + H2
What is 1;2;1;1
The reactants needed to produce the salt iron(II) nitrate, carbon dioxide and water.
What are iron(II) carbonate and nitric acid?
The respective pH of tomatoes and drain cleaner
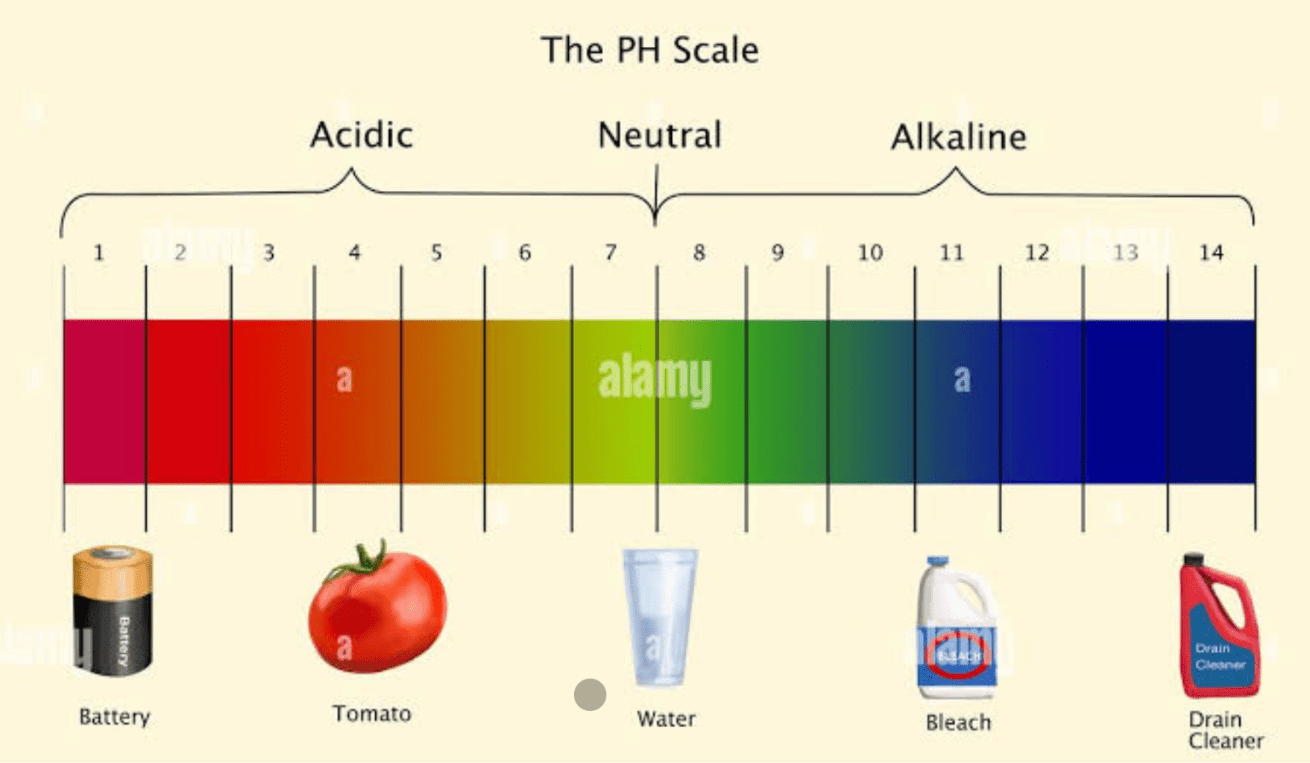
What is 4 and 14?
The number of neutrons in
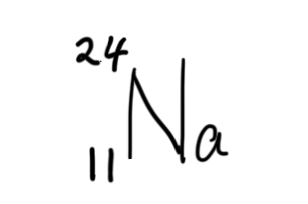
What is 13
The name of this compound, (NH4)2CO3
What is ammonium carbonate?
The coefficients needed to balance the following equation?
C3H8 + O2 —> CO2 + H2O
What is 1:5 :3:4
The precipitate formed when potassium iodide and lead(II) nitrate are mixed. Remember all nitrates are soluble.
What is lead(II) iodide?
The typical pH of a weak acid
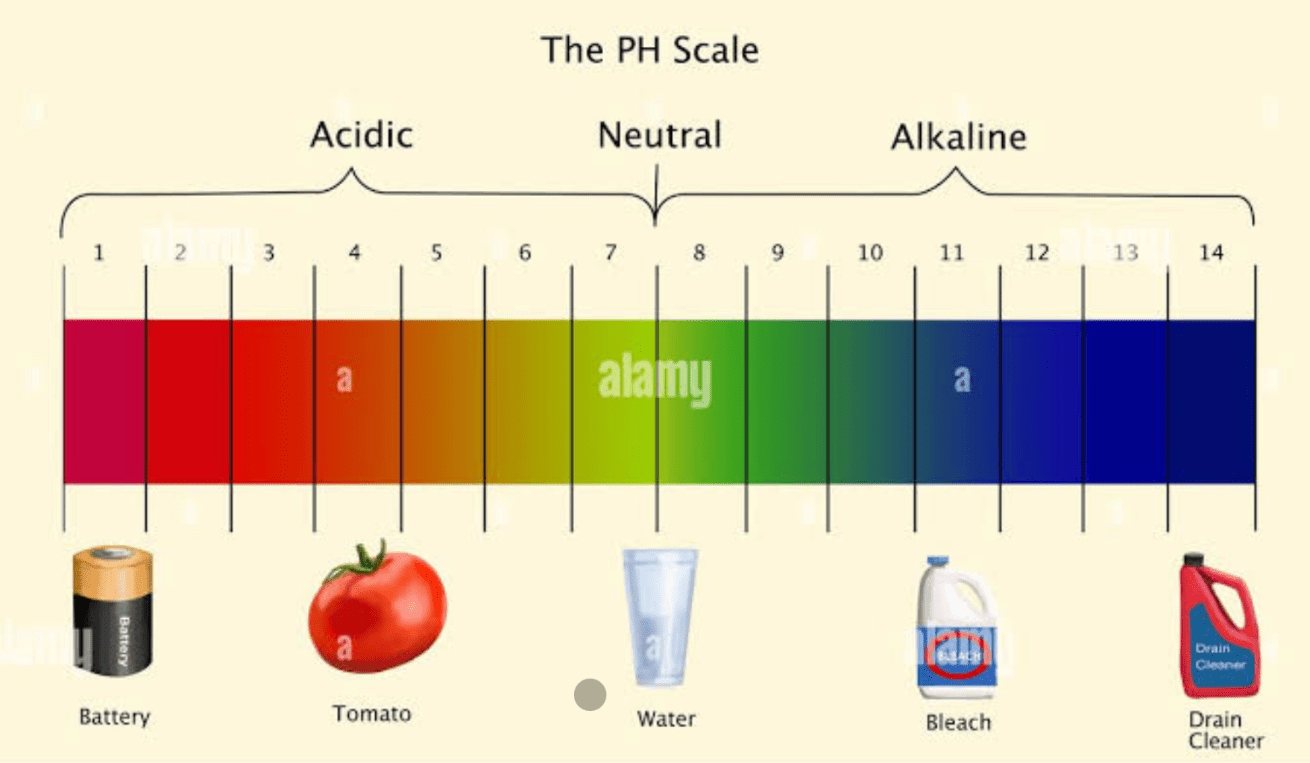
What is 4-6?