What is the name of the following compound:
SF6
Sulfur hexafluoride
(it's not wrong to say "monosulfur" here, just not necessary because it is the first atom listed. If there were 2 sulfurs, you would need to say "disulfur" though)
90.1 degrees Celcius to Kelvin
363.25K
Is baking a cake a physical or chemical change?
Chemical change
How many moles are in a sample of 10.0 grams of silicon? Give correct # sig figs in your answer.
10.0 g Si (1mol Si/28.0855g Si) = .356 moles Si
What's one way to tell the difference between a mixture and a pure substance?
What is this compound called?
HNO2
Nitrous acid
(NO2 is nitrite, -ite -> -ous acid)
How many grams are there in a Megagram?
106 grams or 1 million
A bottle of acetone (nail polish remover) is left uncapped and allowed to evaporate. What type of change has occured?
Physical
You're given a small sample of solid Yttrium of unknown mass. You drop it into a graduated cylinder containing 100. mL of water. You watch the water level rise to 101.9 mL. You know the density of yttrium is 4.472g/cm3.How many milligrams of yttrium do you have? Give your answer in the correct number of significant figures
Volume is 109.1mL - 100.mL = 1.90 mL.
d = m/V
4.472g/mL = m / (1.90mL)
m = 8.50 g
8.50g = 8500 mg = 8.50 * 103 mg
How many neutrons does selenium have?
~45 neutrons
What is this compound called?
NaOH
Sodium Hydroxide
How many electrons does a +3 cation of iron have?
26 - 3 = 23
A cup of milk is left on the counter in Ellie's kitchen for 3 days. (Don't ask me why) What kind of change has occurred if the milk has gone sour?
Chemical
In a cube of solid potassium (density = 0.859 g/mL) with side lengths of 2 inches, how many moles of potassium are present?
1) 2in -> cm; 2in * 2.54cm = 5.08cm sides
2) Vcube = (side length)3
V = 5.083 = 131 cm3
3) Find mass
Note cm3 = mL
d = m/V
0.859 = m/131
m = 113g
4) g -> mol
113 g K * (1 mol K/39.0983g K) = 2.88 mol K
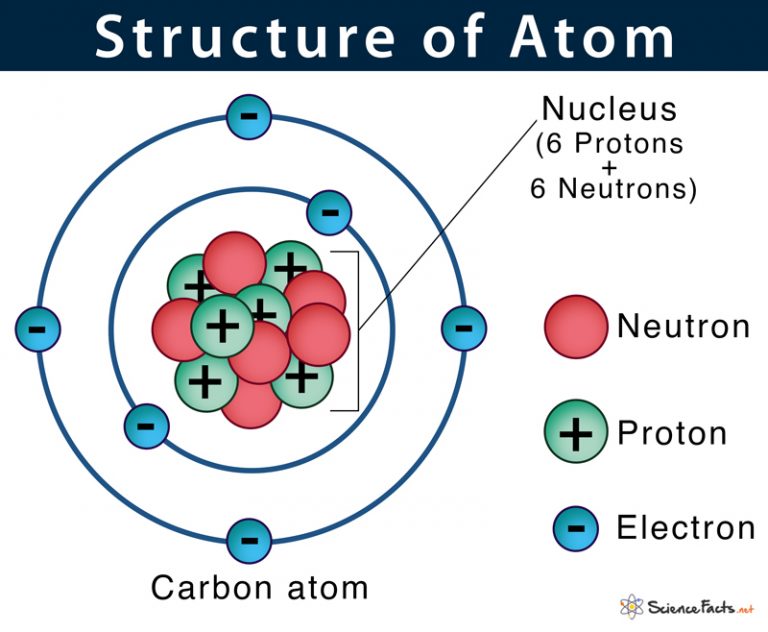
This question has 2 parts. Read carefully...
As fast as you can- draw a diagram of a generic atom.
Diagram must include:
Nucleus, protons, electrons, neutrons.
ALSO, state which part of your diagram would be added or subtracted to create an ISOTOPE of that atom.
Isotopes have more or less neutrons.
What is the name of the following polyatomic ion:
ClO4
Perchlorate
How many protons neutrons and electrons are present in a magnesium ion?
Protons: 12 (atomic #)
Neutrons: 12 (mass # - atomic #)
Electrons: 10 (atomic # - 2 (tendency to form +2 cations))
Is toxicity a physical or chemical property? Is it intensive or extensive?
Chemical, extensive!
Which contains more atoms? 14g of C or 49g of Cr?
14g of C
Which pair of elements would you expect to have similar chemical properties?
A. He and H
B. He and Ne
C. N and O
D. K and Ar
B. He and Ne. Why? They're in the same column!
What is the chemical formula of aluminum acetate?
Al(C2H3O2)3
Thought process... Aluminum forms a +3 cation, and acetate (C2H3O2) forms a -1 anion, therefore we need 3 acetates to make this compound neutral!
Isotopes of antimony are used in the production of medical radioisotopes. There are 2 naturally occurring isotopes of antimony: 121Sb, which has an atomic mass of 120.9038 amu, and 123Sb which has an atomic mass of 122.9042 amu. If 57.21% of all antimony is 121Sb, what is the average atomic mass of antimony?
Calculate weighted averages using the percentages as a decimal
.5721 (120.9038) + .4279 (122.9042)
69.17 + 52.59
121.76 amu with correct sf (2 decimal places)
Is density a physical or a chemical property? Is it intensive or extensive? (Extra 100 points if you can tell me the formula for density)
Physical, intensive. Density = mass/volume (you should know this for your exam on Friday!)
Hydroxyurea is used to treat sickle cell disease by reducing the painful episodes caused by blocked arteries. What percent of Hydroxyurea is nitrogen if the molecular formula for Hydroxyurea is CH4N2O2? Give your answer to 4 significant figures.
N: (14.01 * 2) = 28.02g/mol
Molar mass of hydroxyurea(calculated): 76.055g/mol
% Nitrogen = 28.02/76.055 * 100% = 36.84%
On the whiteboard, draw the outline of a periodic table. Write above each column the type of ion they have a tendency to form. Label the following:
Alkali metals, Alkaline earth metals, halogens, transition metals, noble gases.
Also indicate where you may find nonmetal elements versus metal ones.
You are allowed 1 mistake on this problem for full credit. If completed with no mistakes this question is worth 700 points.
