Groups and Periods

mixture
Physical or Chemical Change:
candle wax melting

physical change
Double Jeopardy!
What elements are in hydroxyapatite, Ca5(PO4)3)OH , a major compound in human bones and teeth?
calcium, phosphorus, oxygen, hydrogen
Do the following represent elements in a group, a period, or neither?
Li, Na, K
Do the following represent elements in a group, a period, or neither?
F, S, P
Neither

element
Double Jeopardy!
Two liquids are mixed and produce bubbles (gas).
Physical or Chemical Change?
chemical change
What is the symbol of the element in Group 2A(2), Period 4
Calcium
Which of the following elements is a metal?
A) nitrogen
B) fluorine
C) argon
D) strontium
E) phosphorus
D) Strontium
A candle wick burning?
Physical or Chemical Change

Chemical change
Double Jeopardy!

mixture
Which of the following are Physical Properties:
1. Density
2. Flammability
3. Reactivity
4. Melting Point
5. Conductivity
Density, Melting Point, Conductivity
Which of the following elements is a noble gas?
A) oxygen
B) chlorine
C) bromine
D) argon
E) nitrogen
D) Argon
The Group 8A(18) elements
A) are unreactive.
B) are good conductors of electricity.
C) melt at high temperatures.
D) are liquids at room temperature.
E) react vigorously with water.
A) Unreactive
Salt Dissolved in water.
Heterogeneous or homogeneous
Homogeneous

Double Jeopardy!
Is the reaction between Mentos and coca cola a physical or chemical change?

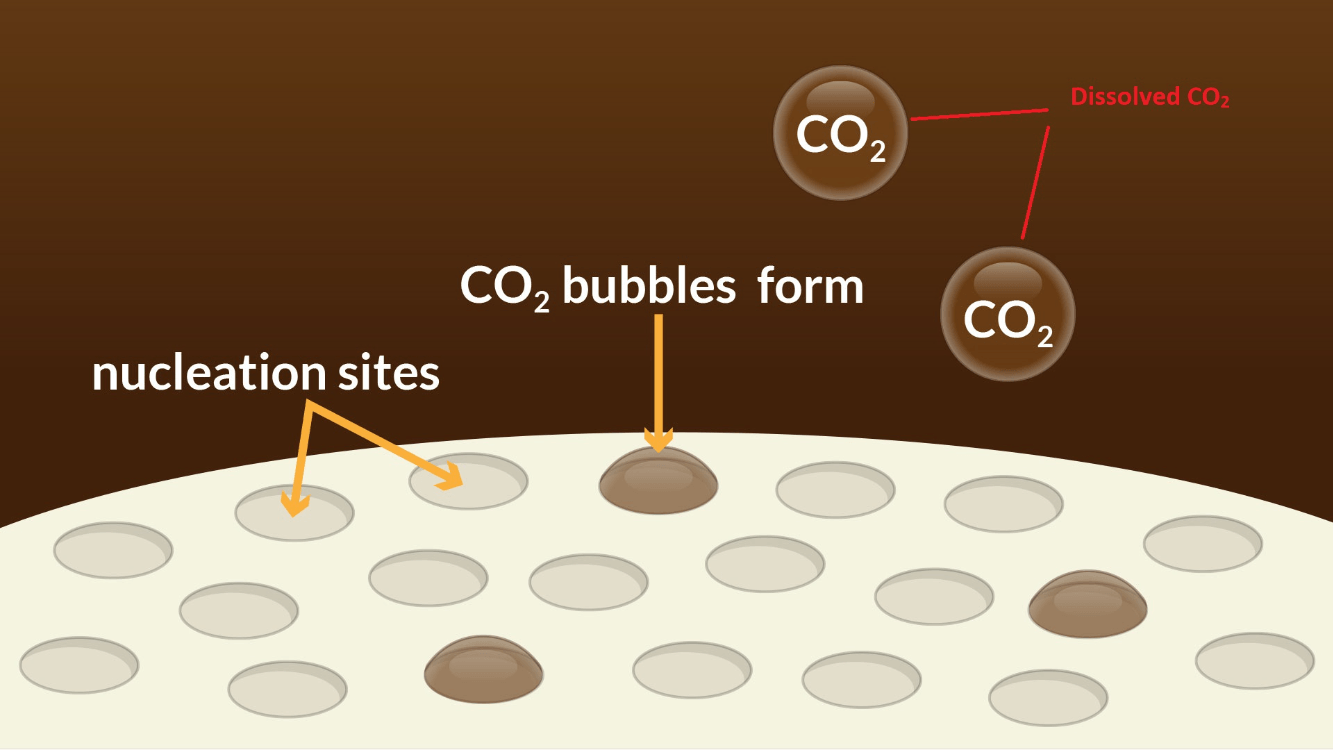
Physical Change: CO2 remains the same compound before and after the reaction.
The element in this list with chemical properties similar to magnesium is ________.
A) sodium
B) boron
C) carbon
D) strontium
E) chlorine
D) Strontium
Which of the following is a characteristic of nonmetals?
A) shiny
B) malleable
C) good conductors of heat
D) low melting points
E) good conductors of electricity
D) Low melting points
Which one of the following substances will float in gasoline, which has a density (d) of 0.66 g/mL?
A) table salt (d = 2.16 g/mL)
B) balsa wood (d = 0.16 g/mL)
C) sugar (d = 1.59 g/mL)
D) aluminum (d = 2.70 g/mL)
E) mercury (d = 13.6 g/mL)
B) balsa wood (d = 0.16 g/mL)
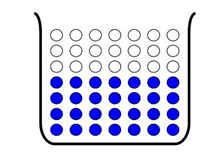
Heterogeneous mixture
Double Jeopardy!
A sample with a mass of 521.0 g is added to 50.0 mL of water. The water level rises to a volume of 77.0 mL. What is density and identity of the sample?

19.3g/mL - Gold
Double Jeopardy!
Which element would have physical and chemical properties similar to chlorine?
A) Ar
B) Br
C) S
D) O
E) P
B) Br
Which of the following properties is NOT a characteristic of the Group 1A(1) elements (alkali metals)?
A) They are shiny.
B) Most of them are liquids at room temperature.
C) They react vigorously with water.
D) They are good conductors of heat.
E) They are good conductors of electricity.
B) Most of them are liquids at room temperature.
Double Jeopardy!
What is the mass of 53 mL of ethyl alcohol, which has a density of 0.79 g/mL?
42 g