What is solid?
Density, volume, and mass are all examples of what kind of properties?
What is physical?
Label each color using the terms: metalloids, metals, and nonmetals. 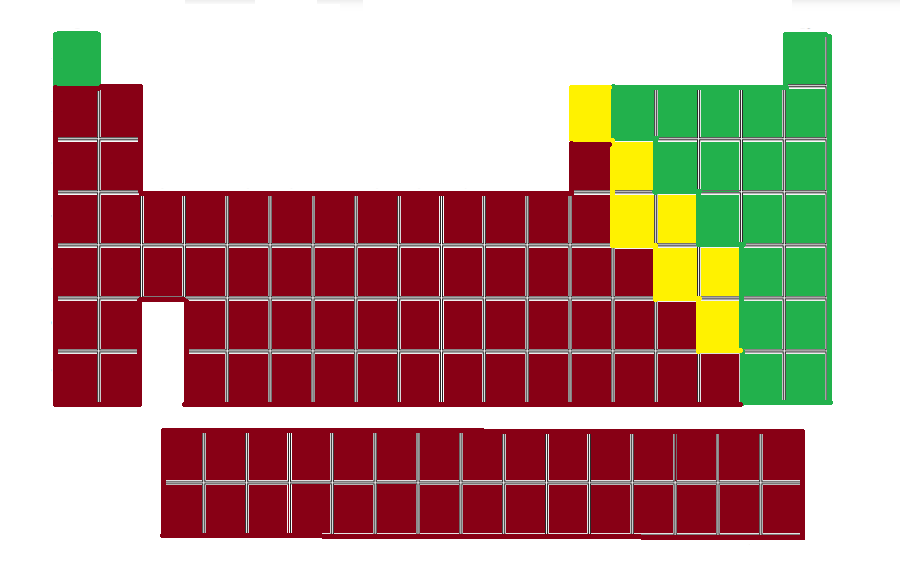
What is...
Green: Nonmetals
Yellow: Metalloids
Red: Metals
The large number shown in front of an element symbol. 2H2
What is a coefficient?
A student has a sample of aluminum that has a mass of 20 g and a volume of 10 cm3. What is the density of aluminum?
What is 2.0 g/cm3?
In this state of matter, particles are close together and are able to slide over each other.
What is liquid?
These properties can only be observed when matter undergoes (goes through) a change to become an entirely new substance.
What are chemical properties?
What type of mixture is this?
What is homogeneous?
The small number to the right of an element symbol. 2H2
What is a subscript?
What is the formula to find density?
In this state of matter, particles float freely.
What is gas?
Acidity, Basicity, Combustibility, Flammability, and Reactivity are all examples of what type of properties?
What are chemical properties?
What type of bond is this?
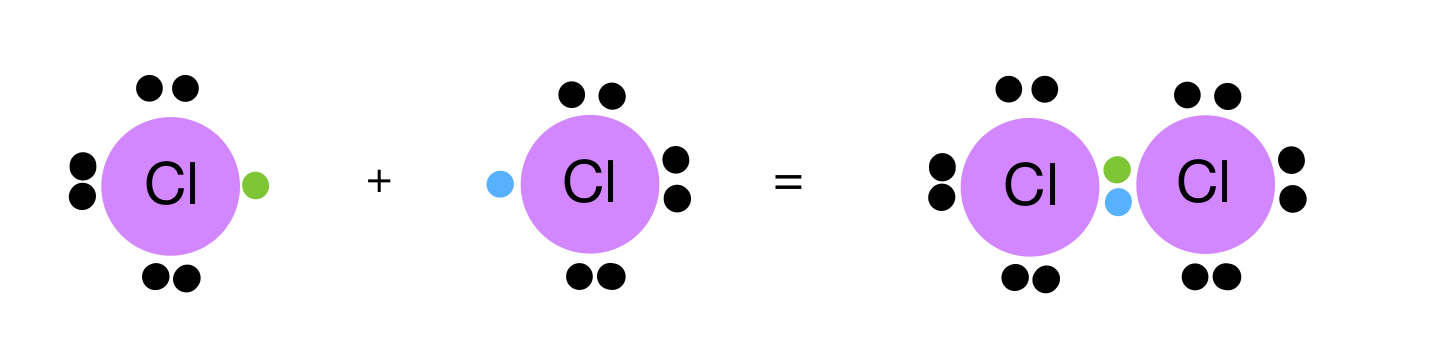
What are Covalent?
When balancing chemical equations, you must change the ___________ and must NOT change the __________.
What are coefficient and subscript?
Calculate the density of sulfuric acid if 35.4 mL of the acid weighs 65.14 g.
What is 1.8 g/cm3?
Out of the three states of matter, this state has the most kinetic energy.
What is gas?
These properties can be observed without changing the make up or composition of matter.
What are physical properties?
Which one represents a mixture?

What is D?
Is the chemical equation N2 + H2 ----> NH3 balanced?
What is no?
What volume of silver metal will weigh exactly 2500 g. The density of silver is 10.5 g/cm3.
What is 238 cm3?
This state of matter can be seen in the Northern Lights.
What is Plasma?
This scale represents the acidity or basicity of matter.
What is the pH scale?
Label each model in order.
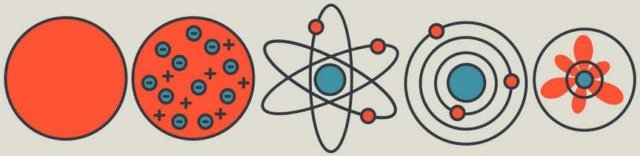
What is Solid Sphere, Plum Pudding, Nuclear, Orbital/Bohr/Planetary, and Electron Cloud.
This law states that matter cannot be created nor destroyed.
What is the law of Conservation of Matter?
A rectangular block of copper metal weighs 1896 g. The dimensions of the block are 8.4 cm by 5.5 cm by 4.6 cm. From this data, what is the density of copper?
What is 8.9 g/cm3?