What is matter?
Anything that has mass and takes up space.
What are the two type of physical properties?
Size-Dependent
Size-Independent
What is a chemical property?
A characteristic of matter that can be observed as it changes to a different type of matter.
What are the three main phases of matter?
solid, liquid, gas
What is it called when a substance changes from a solid to a liquid.
Melting
Steal 300 points from the team in 1st place.
What are the two categories we classify matter into?
Substances
Mixtures
What is a size-dependent property, and give me an example.
Mass
Volume
Give me three examples of chemical properties.
Flammability
Toxicity
Reactivity
Acidity
Heat of Combustion
In which state of matter is the attraction between molecules the strongest?
Solid
Is the following an example of a chemical or physical change:
Ms. Chamberlain burnt her garlic bread in the oven.
Chemical
Choose one team to steal 400 points from.
What are the two categories of mixtures, and what is the difference between the two?
Heterogeneous: two or more substances unevenly mixed.
Homogeneous: two or more substances evenly mixed.
What is a size-independent property, and give me three examples.
Does not Depend on the amount of matter present.
Melting point, boiling point, density, conductivity, solubility, state of matter, magnetism.
Is blowdrying your wet hair an example of a physical or chemical change?
Physical
In which state of matter are molecules moving at the fastest speed?
Gas
What is the name of the county Ms. Chamberlain grew up in.
Mineral County
Plus 500
What are the two categories of substances, and what is the difference between the two?
Elements: a substance that consists of just one type of atom.
Compounds: Two or more types of atoms bond together.
Give me three examples of a physical change.
Chemical
What is plasma?
When matter is heated to a temperature over 5000 C, the collisions between atoms become so violent that electrons are knocked away from the atoms.
What is called when you go from a liquid to a gas?
Evaporation or vaporization.
switch places with the team in 3rd place.
The following picture is an example of which type of substance or mixture?
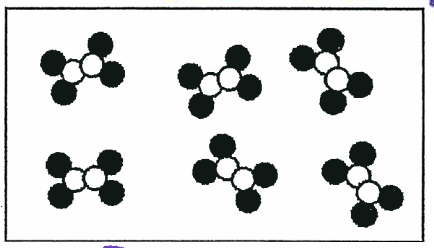
Compound
Describe what would happen to a persons mass and weight if they were on the moon.
Mass would not change, weight would change.
1. Tell me the elements that make up this chemical formula.
2. Tell me how many of each element are on the product side, then the reactant side. 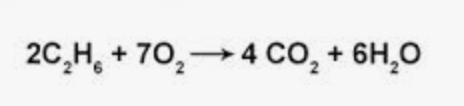
Carbon, Hydrogen, Oxygen
4 Carbon
12 Hydrogen
14 Oxygen
Describe the shape and volume of each of the 3 states of matter.
Solid: definite shape and volume.
Liquid: Definite volume, takes on shape of container.
Gas: No definite shape or volume.
In what city was Ms. Chamberlain born in?
Helena
Add 600 points to your score