Moles + Conversions
Solutions + Concentration
Equilibrium
Thermodynamics+Kinetics
Lies + Lab
100
Which is heavier: 20 grams of iron (Fe), or 20 grams of nickel (Ni)?
 _
_
TRICK QUESTION (neither)
100
This is the measure of how much an ion or compound will dissolve into solution.
What is SOLUBILITY?
100
This indicates the ratio of products to reactants of a given reaction at equilibrium.
What is the equilibrium constant (Keq)?
100
This kind of reaction releases heat into the environment.
What is an EXOTHERMIC reaction?
100
These are individuals that appear as authorities to a subject without relevant expertise (including celebrity endorsements).
What are PSEUDO EXPERTS?
200
This is the molecular mass of fluorine (F2).

What is 38 g/mol? (19 + 19)
200
This is the mass concentration (g / L) of 100 g of NaHCO3 in 2 L solution.
What is 50 g / L?
200
Increasing the pressure on the following reaction will cause a shift in this direction.
2SO2(g) + O2(g) ⇄ 2SO3(g)
What is a shift to the RIGHT (towards products).
200
This is a chemical that can speed up the rate of a reaction by lowering the activation energy.
What is a CATALYST?
200
Water was used as this kind of control in the nutrients lab.
What is a NEGATIVE control.
300
This is the molecular mass of carbon dioxide (CO2).
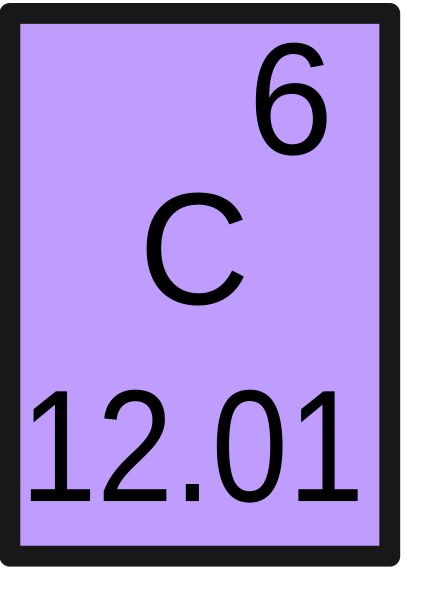 _
_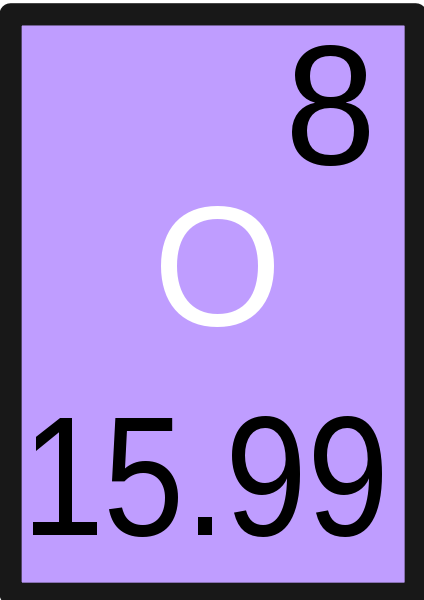
What is 43.99 g/mol? (12.01 + 15.99 + 15.99)
300
This is the molar concentration (mol / L) of 4 mol of NaCl in 2 L solution.
What is 2 mol / L?
300
An increase in temperature will usually cause exothermic reactions to shift in this direction.
What is a shift to the LEFT (towards reactants).
300
This is one way to speed up an endothermic reaction.
What is apply heat? (also: increase concentration of reactants or add a catalyst)
300
This is the tendency to interpret and favor information in a way that confirms one's beliefs or hypotheses.
What is CONFIRMATION BIAS?
400
DAILY DOUBLE!

400
This is the molar concentration (mol / L) of 13 mol of sucrose in 0.5 L solution.
What is 26 mol / L?
400
Removing products of a system at equilibrium will shift the reaction in this direction.
What is a shift to the RIGHT (towards products).
400
This is the minimum energy required for a reaction to happen.
What is the ACTIVATION ENERGY (Ea)?
400
During the "elephant toothpaste" demonstration, this was the role of the potassium iodide solution.
What is CATALYST (lower Ea and speed the reaction)?
500
About this many atoms can be found in a 28 g chunk of iron.

(1 mol = 6.02 x 1023 items)
What are 3.01 x 1023 atoms? (0.5 mol Fe)
500
DAILY DOUBLE!

500
When Q > Keq, the reaction will shift in this direction to reach equilibrium.
What is a shift to the LEFT (towards reactants).
500
This is a measure of disorder, and is what makes endothermic reactions possible.
What is ENTROPY?
500
This principle states that any technology with the potential to harm should NOT be introduced until proven completely safe.
What is the PRECAUTIONARY PRINCIPLE?