The three common states of matter
Who established the formula below: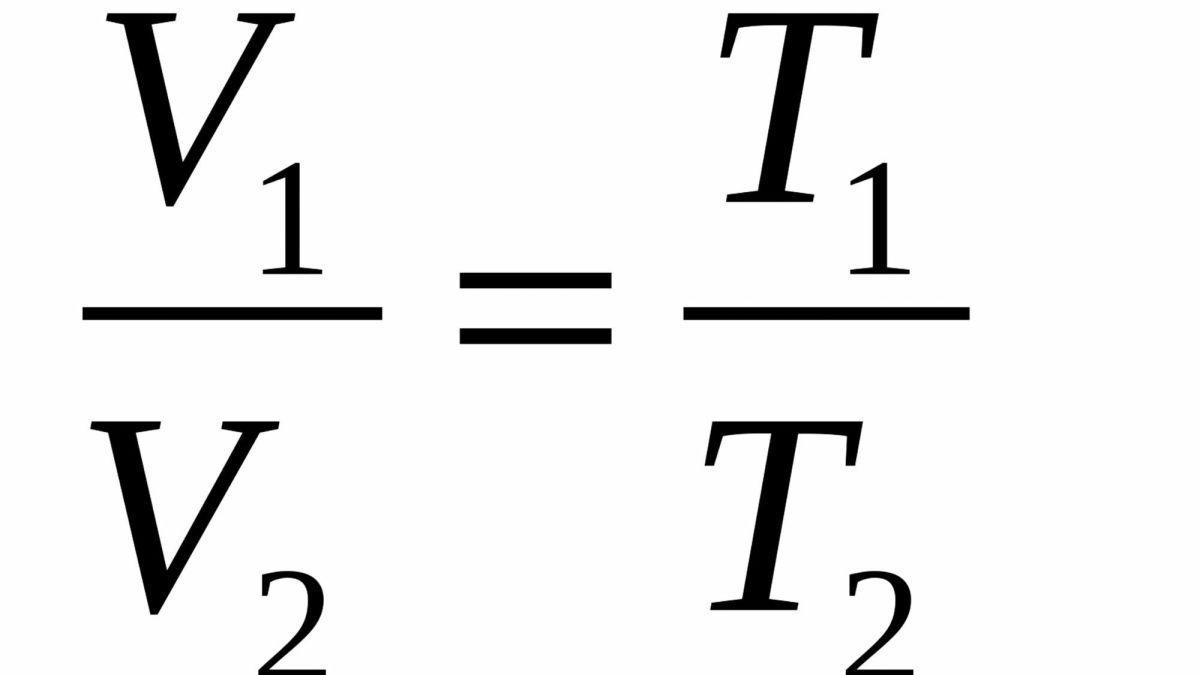
Who is Jacques Charles?
True or False: We can solve gas law formulas by using degrees Celsius.
What is false?
The point at which a liquid becomes a gas.
What is boiling point?
The negative particles of an atom.
State of matter represented by this image:
What is solid?
What the "P" stands for in Gay-Lussac's Gas Law below: 
What is pressure?
What we have to do to degrees Celsius to solve a gas law formula involving temperature.
What is convert (to Kelvin)?
Water is called this when it becomes a solid.
What is ice?
The term for how fast or slow a liquid flows.
What is viscosity?
State of matter represented by this image:

What is gas?
The chemist who established the gas law below:

Who is Robert Boyle?
The number we have to add to convert from Celsius to Kelvin.
What is 273.15?
The point at which a solid becomes a liquid.
What is melting point?
What does NaCl stand for?
What is salt or sodium chloride?
The way in which particles of a solid move.
Boyle's Law describes the _______ relationship between the pressure and volume of gas at a constant temperature. If pressure increases, volume decreases.
What is inverse?
30 degrees Celsius to Kelvin
What is 303.15K?
Water is called this when it reaches its boiling point.
What is steam?
What contains the known elements?
What is the Periodic Table of Elements?
The particles in this state move freely or slide past one another.
What is liquid?
Law that states when pressure on a fixed amount of gas is held constant, its volume is directly proportional to its absolute temperature in Kelvin.
What is Boyle's Law?
75 degrees Celsius to Kelvin
What is 348.15K?
The point at which a liquid becomes a solid.
What is freezing point?
What is exothermic?