Which is true about atoms?
a) Atoms of any given element will differ from those of other elements
b) An atom will always display a positive charge
c) An atom will always display a negative charge
d) Atoms are made up of elements
A
Name 2 difference between ionic and molecular substances?
- Molecular compounds are electrically neutral while ionic compounds for ions
- Ionic compounds have transferred electrons while molecular has shared electrons
- Ionic compounds tend to form lattice structures making them primarily solids, while molecule compounds can come in any state.
Which in the following pair has the SMALLEST atomic radius?
P or O
O, Oxygen
What equation is used to convert between moles, mass and molar mass.
n= m/M
Name the following compound:
NaBr
Sodium bromide
True or False
Ions are always negatively charged
False,
An ion is any atom that has gained or lost electrons changing its charge from neutral to either positive or negative.
Draw the lewis structure for CO2
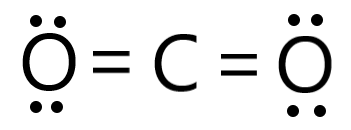
Which element in the pair below has the larger ionization energy?
Mg or F
F
What equation is used to convert the number of moles to the number of atoms/molecules or units?
n = N/(N_A)
Name the following compound:
PbO2
Lead (IV) Oxide
Remember: Oxygen has a "charge" of 2 since, lead has no subscript on it, there must have been some sort of reduction!
What is the difference between an ion and an isotope?
Isotopes are a different number of neutrons (No change to over all charge)
Ions are a different number of electrons (changes charge)
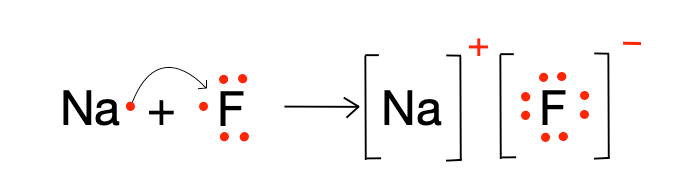
Draw the lewis structure for NaF
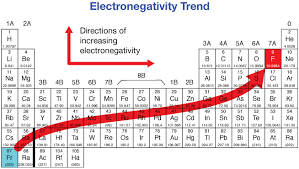
Which of the following has the highest electronegativity?
Ge
O
P
O, oxygen
What is avogadro's number?
6.02x10^23
What would be the formula for the following compound?
sulfur hexafluoride
SF6
No prefix on sulfur means 1, hexa- means 6.
How is the atomic mass of an atom developed?
It is a weighted average of the masses of the elements isotopes based on their abundances.
If potassium has an electronegativity of 0.82 and sulfur has an electronegativity of 2.58 what type of bonding is likely to occur?
Ionic
2.58 - 0.82 = 1.76
These elements do not react easily with anything as they are already stable
Noble gases
How many molecules are in one mole of water?
6.02x10^23
If you are not given a total mass or molar mass when completing the empirical formula, what must you assume before you start?
That you have 100g of the substance (this makes it easier when working with the percentages).
The average atomic mass of magnesium is 24.305u. The element has three natural isotopes:
magnesium-24
magnesium-25
magnesium-26
Which of the isotopes is likeliest to be in the greatest abundance?
magnesium-24
Since the average mass is 24.305u, this is closest to the isotope of magnesium-24; therefore it is most likely in the greatest abundance.
What is the name of the forces that hold the molecules in molecular compounds together?
Intermolecular forces
An element is a metal and reacts very easily with water. What family is this element most likely in?
Group 1 elements, the alkali metals
How many atoms are in 3 moles of carbon?
1.81x10^24
What is the main difference between an empirical formula and a molecular formula?
Empirical is the simplest whole-number ratio (its reduced to its lowest form)
Molecule formulas show the accurate number of each atom type in a molecule.