An oceanic plate slides under a continental plate (like what is happening off the coast of WA). What type of plate boundary is this?
Convergent
A substance that is only made up of one type of atom and cannot be broken down into a simpler substance is called.
Element
What are the base measurements we used?
Gram, Meter, Liter
Is a jar of different types of candy an example of a homogeneous or a heterogeneous solution?
Heterogeneous
A mineral sample has a mass of 116.3 g and a volume of 14.35 cm3. What is its density?
8.1 g/cm3
When an earthquake happens what is the name for the spot on the EARTH's surface for where the quake starts?
Epicenter
What two subatomic particles are found in the nucleus?
Protons (+) and Neutrons (0)
What is the metric prefix that means 1,000?
Kilo
What is malleability?
Which type of earthquake wave travels the fastest?
P wave
In sea floor spreading what is happening at the mid ocean ridge?
New crust is being created
How do you find the number of neutrons in an atom?
Atomic mass (rounded) - atomic number = Neutrons
1.457 Km = ______ m
1,457 Meters
How do we describe a gas in terms of shape and volume.
No definite shape or volume
What does SONAR use to map the ocean floor?
Sound waves
Name the three major types of rock?
Sedimentary, Metamorphic, Igenous
If an element loses an electron what does its charge become? What do we now call that atom?
+1, Ion
What is the prefix name for µ
micro
In what phase of matter is the attraction between the molecules the strongest.
Solid
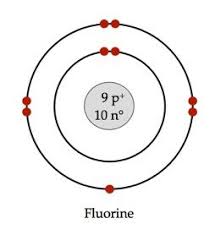
Draw the Bohr Moodle for Flourine
Name the three pieces of evidence that support the idea of continental drift.
1. Fossils on different continents
2. Matching mountain ranges
3. Continents fit together like puzzle pieces
What happens in an ionic bond versus what happens in a covalent bond?
Ionic bonding is the transfer of electrons whereas a covalent bond is sharing electrons
45 Hm = _______ cm
450,000 cm
The phase change in which a gas changes directly into a solid is called.
Deposition
I am roasting marshmallows and starting at the campfire. I begin to wonder, is the wood burning and creating smoke a physical or a chemical change?
Chemical Change