Matter cannot be ______________ during a chemical reaction.
a) B and C
B) destroyed
C) created
D) changed
a) B and C
How many total atoms of Chlorine are there in 2NaCl2 ?
4
If a chemical reaction begins with 6 atoms of carbon (C), how many atoms of carbon (C) should be in the products?
6 atoms of Carbon (C)
Jackie and Josh measured 35g before and 33g after mixing baking soda and vinegar. What explains the missing mass?
a) Gas was released and not captured
My Science teacher is
Ms. Rosales
The total mass of the products of a chemical reaction _______ the total mass of the reactants.
is the same as
Which chemical equation supports the Law of Conservation of Mass?
a) 2Al + 3HCl → 2AlCl₃ + 3H₂
b) SO₂ + O₂ → S₂O₅
c) 4P + 4O₂ → 2P₂O₅
d) 2H₂O → 2H₂ + O₂
d) 2H₂O → 2H₂ + O₂
What number represents a coefficient in the products in this equation: Cl₂ + Na → 3ClNa?
3
What is the importance of a closed container in a chemical reaction?
to observe the mass before and after the reaction occurs
What is the capital city of France?
Paris
How does a balanced chemical equation satisfy the Law of Conservation of Mass?
During a chemical reaction, the total amount of matter stays the same
3P + 5O₂ → P₂O₅. Is it balanced?
balanced or unbalanced?
explain why?
unbalanced
Which equation is balanced according to the Law of Conservation of Mass?
a) 2NaCl + Mg → MgCl₂ + Na
b) 2NaCl + Mg → MgCl + 2Na
c) NaCl + Mg → MgCl + Na
c) NaCl + Mg → MgCl + Na
A 10g aluminum can reacts with 100g hydrochloric acid (total mass 110g) , after the reaction the mass was 108g. What explains the missing 2g?
Mass lost as gas
Purple
Which best explains why the total mass of the product(s) would be less than the total weight of the reactant(s) after a chemical reaction?
a) Atoms involved in the reaction lost mass.
b) Precipitates were created in the new solution.
c) A physical change occurred.
d) Gases were released to the atmosphere.
d) gases were released to the atmosphere
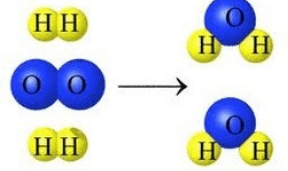 Which equation supports this model?
Which equation supports this model?
a) 2H2O2 → 2H2O b) 2H2 + O2 → H2O
c) 2H2 + O2 → 2H2O d) H2 + O2 → 2H2O
c) 2H2 + O2 → 2H2O
If a chemical equation is not balanced, what does that mean about the law of Conservation of Mass?
The number of atoms on both sides is not equal, so mass is not conserved
When you bake bread, yeast makes gas that make the dough rise. Why does the dough's weight stay about the same?
The dough is in a closed oven, so nothing escapes.
How many colors are in a rainbow?
7
When vinegar and baking soda are combined, a mixture is produced, and gas is released. Which statement is true?
a) The mass of the gas is equal to the mass of the vinegar and baking soda
b) The mass of the mixture and gas is greater than the mass of the baking soda and vinegar
c) The mass of the mixture and gas is equal to the mass of the vinegar and baking soda
a) The mass of the gas is equal to the mass of the vinegar and baking soda
According to the Law of Conservation of Mass, what will be the mass of Zinc after the reaction has taken place?
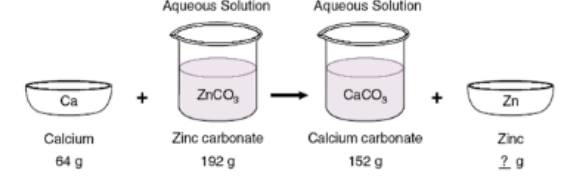
104 g
Why is it important for a chemical equation to be balanced?
To show the same number of atoms on both sides, obeying the law of conservation of Mass
Fireworks explode in the sky making light and gas. Why might the weigh of the fireworks before and after the explosion be different if measured outside?
Some gas and smoke float away into the air
Who painted the Mona Lisa?
Leonardo da Vinci