Matter cannot be __________ or __________.
Created, Destroyed
The arrow is pointing to it.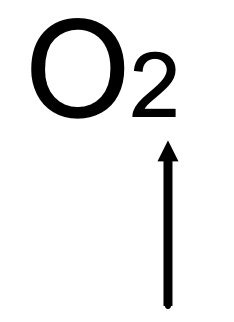
What is Subscript?
What is the missing coefficient?
2H2 + O2 ---> _____ H2O
2
Valence electrons are shared or transferred to form chemical _________.
Bonds.
3H2
The number of Hydrogen atoms.
What is 6?
H2
What is Covalent
The chocolate chip cookie was invented in 1938. When was the Oreo invented?
For something to be considered a chemical change, a _____________ substance must be created.
Matter can only change _____ or______
Shape or form
The arrow is pointing to it.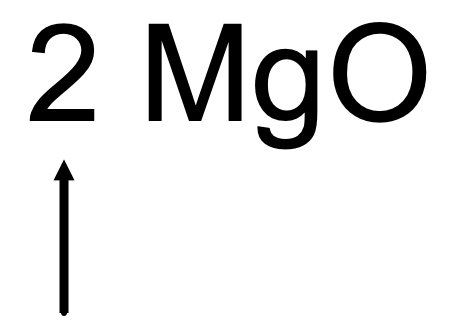 .
.
What is Coefficient?
What is the missing coefficient?
___Na + O2 ---> 2Na2O
4
When valence electrons are transferred (gained or lost).
What are Ionic Bonds?
4HCl
The number of Chlorine atoms.
What is 4?
CO2
What is Covalent
The team who won the SuperBowl during the 2005-2006 season.
Who are the Pittsburgh Steelers?
In a physical change, a substance only changes in ______________.
A __________ chemical equation follows the Law of Conservation of Matter.
Balanced
The small red arrow is pointing to it.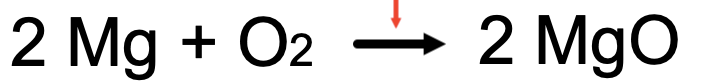
What is Yield Sign?
What is the missing coefficient?
2Al2O3 ---> 4Al + ____O2
3
When valence electrons are shared.
What are Covalent Bonds?
4NaO2H2
The number of Hydrogen atoms.
What is 8?
Be2C
What is Ionic
If you were to fight every person in the world in a March Madness style bracket, how many people would you have to fight to be champion of the world? (How many rounds to the bracket?)
33
Identify the process as a chemical or physical change.
Dissolving Salt into Water to make Saltwater.
What is Physical?
In chemical equations, the sum of the masses of the reactants is _______ to the sum of the masses of the products.
Equal
The compounds found on the left side of a chemical equation.
What are reactants?
What are the missing coefficients?
____Fe + 4H20 ---> Fe3O4 + ____H2
3, 4
The closer atoms are to having 8 valence electrons, the more ___________ they are.
5Mg2O2
The total number of atoms in the chemical formula.
What is 20?
Fr2S
What is Ionic
The amount of time the value Pi (π) has been known for. (how long ago it was discovered)
4,000 years
Identify the process as a chemical or physical change.
A plant preforming Photosynthesis.
Chemical Change
A Product in the chemical equation.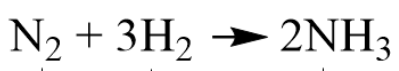
What is 2NH3?
The compounds found on the right side of a chemical equation.
What are Products?
What is the missing coefficient?
2C2H6 + ____O2 ---> 4CO2 + 6H2O
7
The purpose of chemical bonding is so that atoms can become ________. (the answer is not happy, use the scientific term).
Stable
2Na3PF4
The total number of atoms in the chemical formula.
What is 16?
HCl
Covalent
The amount of time Dinosaurs were actually seen on screen during the original Jurassic Park movie.
What is 15 minutes?
In an Exothermic Reaction, energy ___________ the reaction. These reactions often feel ____________. One example is ___________.
Exits, Hot/Warm, Fire
Identify a Reactant in the Chemical Equation.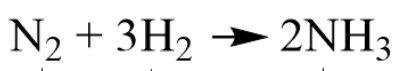
What is N2 or 3H2?
The subscript tells us the number of __________ for each element while the Coefficient tells us the number of ___________.
Atoms, Molecules
What are the missing coefficients?
____HCl + ____Na ---> ____NaCl + H2
2, 2, 2
We discussed 5 signs that a possible chemical change has occurred. List 3 of those signs.
Bubbling / Fizzing, Unintentional Color and/or Temperature Change, Smoke / Fire, Formation of a Solid (precipitate)
4Ca2(OH)4
The total number atoms in the chemical formula.
What is 40?
NaKSe
Ionic
What is Napoleon Bonaparte's (French military leader) win percentage in major battles?
88%. He fought in 60 battles and lost only 7.
In an Endothermic Reaction, energy ___________ the reaction. These reactions often feel ____________. One example is ___________.
Enters, Cold, Cooking Food