What is 22?
Elements that are located in the same column with similar chemical properties are called this.
What is a group or family?
This element is found at period 4, Group 8
What is FE or iron?
The large number in front of the following molecule represents this. 3H2O
What is the number of molecules?
This represents the total number of molecules present of that particular element or compound.
What is the coeeficient?
Number of valence electrons in the following atom.
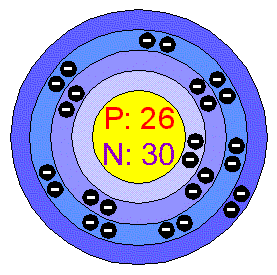
What is 2?
This is the number at the top of the element.
What is the atomic number?
This element is in group 3, Period 2
What is Magnesium?
The number 2 in this molecule is called this.
3H2O
What is the subscript?
This is Mrs. Atwood's favorite college football team.
What is Notre Dame?
This shell of an atom can only have two electrons in it.
What is the first?
The three main categories on the periodic table.
What are metals, non-metals, and metalloids?
The symbol for this element is NA.
What is Sodium?
This molecule has 8 __________ atoms.
(NH4)2SO4
What is Hydrogen?
This is Mrs. Atwood's favorite college basektball team.
What is Indiana University?
There are a total of this many atoms in an ammonium sulfate, (NH4)2SO4 atom.
What is 15?
Zurchonium is located here on the periodic table.
What is period 5, group 4?
This element is the most reactive on the periodic table.
What is Fluorine?
This is a group of two or more atoms bonded together.
What is a molecule?
This is the date that St. Patricks Day will fall on in 2025.
What is March 17th?
This number of valance electrons makes an element "happy" of stable.
What is 8?
This is what elements in the same group have.
What are valence electrons or electrons in the outer shell?
This is the least reactive element on the Periodic Table
What is helium?
This is a molecule with elements always in the same ratio.
What is a compound?
Early in earth's history hydrogen combined with carbon to make this molecule.
What is a hydrocarbon?