Is H2O polar or nonpolar?
Polar
In covalent bonds, electrons are ________?
What is Shared
A substance required for a chemical reaction to occur; found before the arrow
What is a reactant?
This is the correct name for water.
What is Dihydrogen Monoxide?
What Type of Reaction is this: Synthesis, Decomposition, Single Replacement, Double Replacement, or Combustion?
__ H2 + __Cl2 --> __HCl
What is Synthesis?
Non-polar Substance have (equal or unequal) sharing of electrons
equal
Which diatomic element has a triple bond between its atoms?
What is Nitrogen?
The coefficients in a chemical equation represent this.
Number of moles / mole ratios
The prefix for four is this.
What is Tetra?
You mix two substances in a test tube. The test tube gets colder. Exo or Endothermic?
What is endothermic?
When there is an unequal sharing of electrons the substance is said to be polar or nonpolar?
What is polar
We represent one shared pair of electrons with this number of lines drawn between 2 atoms.
What is 1.
State the Law of Conservation of Mass
What is "matter is not created nor destroyed in a chemical reaction"?
The correct name for this compound, N2O2, is this.
What is Dinitrogen Dioxide
Is the following reaction endothermic or exothermic?
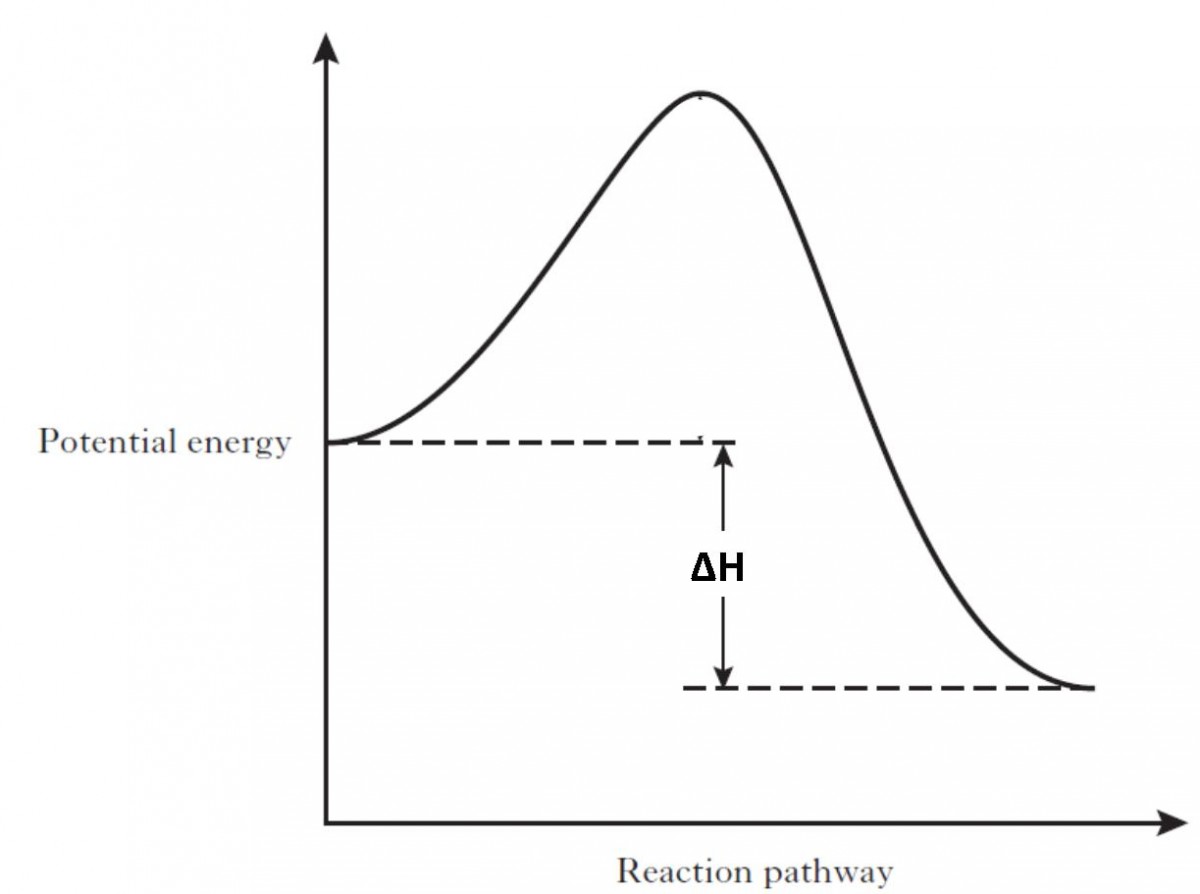
What is Exothermic
Identify a binary molecular compound that is nonpolar.
What is CO2/CH4/other answers may be acceptable
Molecules refer to compounds that have this type of bonding.
What is Covalent?
Balance the Equation:
____ NH3 + ____ O2 → ____ NO + ____ H2O
_4_ NH3 + _5_ O2 → _4_ NO + _6_ H2O
The prefix for 9 is this.
What is nona?
Classify:
2Al2O3 --> 4 Al + 3 O2
What is decomposition?
When a molecule is polar it is said to have this: _____, meaning both a partial positive and partial negative side.
What is a dipole?
When an atom of F attracts shared electrons more than an atom of H, we say F is more _____________ than H.
What is electronegative?
The amount of activation energy needed to start this reaction:
What is 200 kJ?
P5Cl7 takes this name.
What is pentaphosphorous Heptachloride?
The type of reaction shown here:
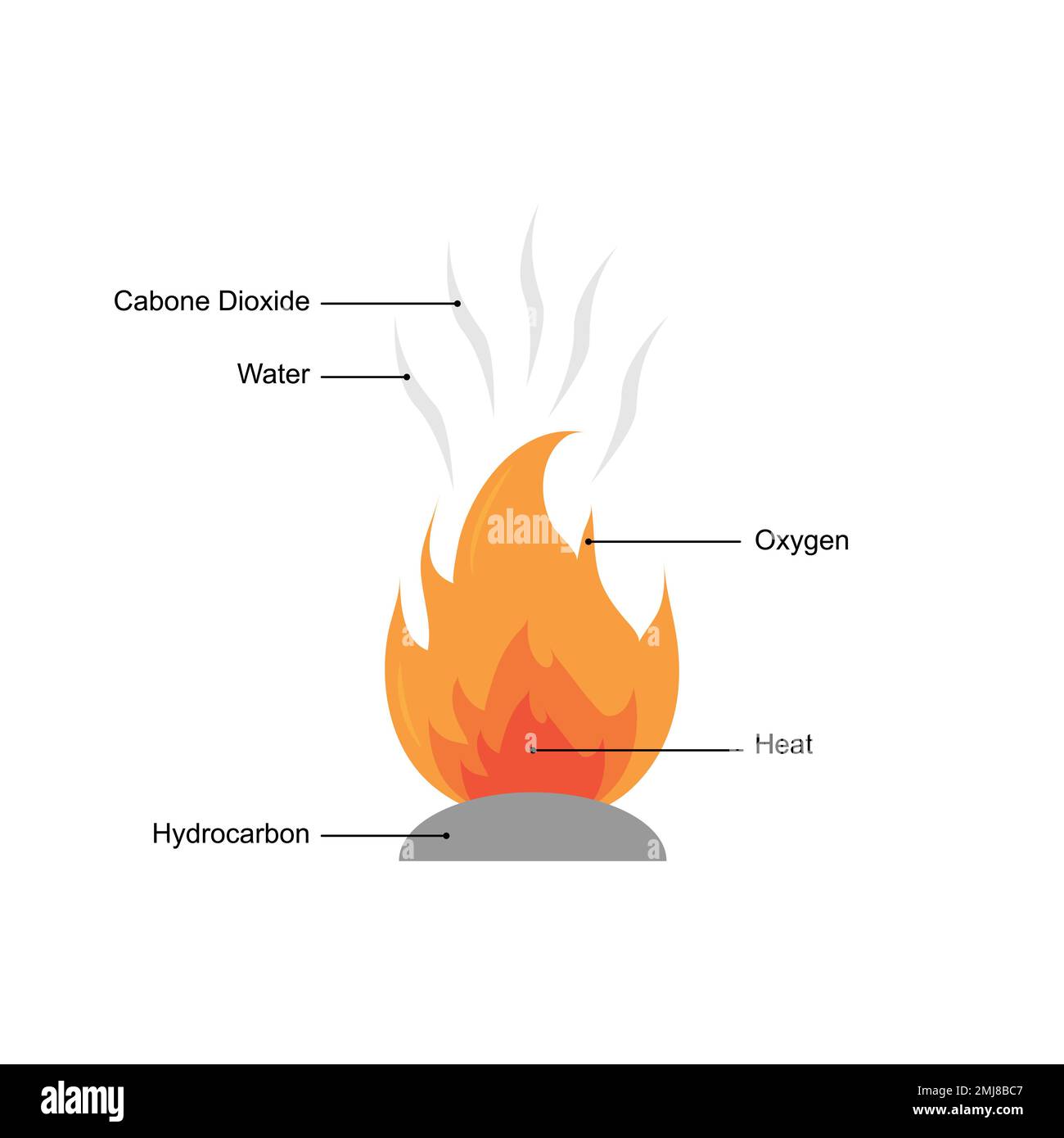
What is combustion?