Write the name of the following molecule:
NI3
Nitrogen Tri-iodide
Draw the Lewis structure for CCl4

What is the difference between a polar and nonpolar covalent bonds?
Nonpolar shares electrons equally, polar shares electrons unequally
Determine the molecular geometry of the molecule below:
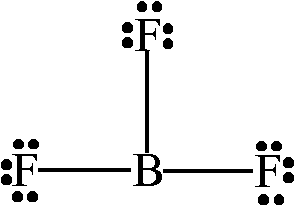
Trigonal Planar
Put the following IMFs in order from WEAKEST to STRONGEST: dipole-dipole, ion-dipole, London dispersion forces and hydrogen bonding
London Dispersion Forces, Dipole-Dipole, Hydrogen Bonding, Ion-Dipole
Write the chemical formula for the compound:
silicon tetra bromide
SiBr4
Draw the Lewis structure for NF3

Is this bond ionic, polar or non polar?
H-Cl
Polar
Determine the molecular geometry of the molecule below:

Bent
What type of intermolecular forces do non polar molecules have?
London Dispersion Forces
Write the name of the following molecule:
PF5
Phosphorus Pentafluoride
Draw the Lewis Dot structure for BeCl2
(incomplete octet)

Is this bond ionic, polar or non polar?
C-H
Determine the molecular geometry of the molecule below:

Trigonal Pyramidal
What types of intermolecular forces does PBr3 have?
London Dispersion Forces and Dipole-Dipole
Write the chemical formula for the compound:
Xenon nonafluoride
XeF9
Draw the Lewis structure for PCl5
(Expanded Octet)
Is H2O a polar or nonpolar molecule?
Polar
Determine the molecular geometry of the molecule below:
Tetrahedral
What types of intermolecular forces does NH3 have?
London Dispersion Forces, Dipole-Dipole and Hydrogen bonding.
Write the name of the following molecule:
P4O10
Tetraphosphorus decaoxide
Draw the Lewis structure for CO2
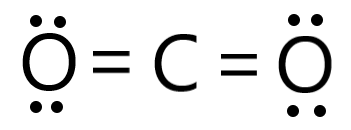
Is CCl4 a polar or nonpolar molecule?

Nonpolar
Determine the molecular geometry of the molecule below:
See-Saw
Which molecule will have the higher boiling point: CF4 or H2O?
H2O