Covalent Bond
The sharing of electrons
Draw HI
. .
H-I..
. .
If there's net movement then the molecule is what?
Polar
What's it mean to be non-polar?
Even sharing of electrons
Draw a Lewis Dot Structure for N
5 dots around N
Why are some atoms only in compounds of paired with another atom of the same element?
Because they're more stable together than they are alone
Draw a line structure of H2O and give the molecular shape.
Bent
Is N or O more EN and why?
O because it's further to the right on the PT
Why is water polar?
Unequal sharing of electrons or net movement!
How could you test an unknown compound to see if it was a covalent compound?
Test for low melting/boiling points, gas or liquid at room temperature, soft unless in a network then hard and brittle, nonconductors of electricity and heat
Lithium has 1 valence electron. What would this atom do to create a full octet?
Lose the electron to make a Helium like electron configuration.
Draw a line structure of CH2O
..O..
||
H - C - H
Using your PT with electronegativity values, what kind of bond forms between C and O
3.44 (O) - 2.55 (C) - 0.89 so polar covalent!
Is CH4 a polar or non-polar molecule? What shape is it?
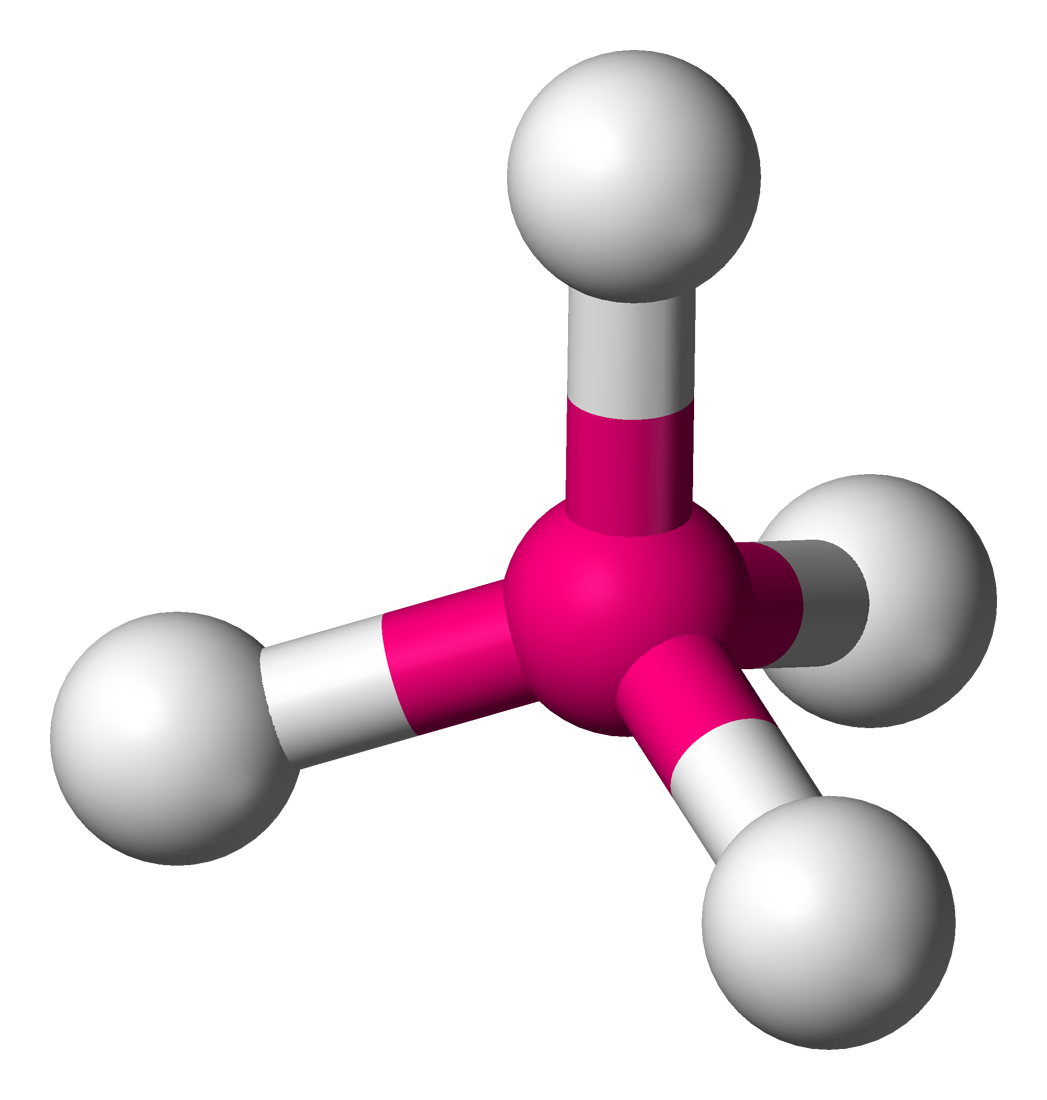
Non-polar, tetrahedral
Name the following: Si7F
heptasilicon fluoride
Define resonance.
When a compound has more than 1 correct Lewis Structure
Draw CSO
. . . .
S = C = O
. . . .
Using your PT with electronegativity values, what kind of bond forms between H and I
2.66 (I) - 2.2 (H) = 0.44 means covalent
Is COS polar or non-polar? Draw a line structure as evidence.
O <- C -> S
Non-polar because there's no net movement!
What's the term for the special 7 elements who like to be bonded to each other.
Diatomic Molecules
Why do we care about VSEPR models?
Since electrons are all negative the bonds and lone pairs repel each other creating their molecular shapes
Draw a line structure and give the molecular shape of PBr3
. .
Br - P - Br
-
Br
trigonal pyramidal
What's the most EN atom on the PT? What does that mean?
Fluorine because (minus the noble gases) it's the most top right atom. EN means how good elements are at attracting/holding onto electrons.
Is CH2O polar?
Polar
Name the following:
H2CO3
carbonic acid