Convert 456 kPa to Torr
3,420.28 Torr
Which of the following processes are exothermic? Select all that apply.
condensation
melting
boiling
deposition
sublimation
condensation
deposition
Which of the following regions of the electromagnetic spectrum has the longest wavelength?
blue light
x rays
infrared
microwaves
orange light
microwaves
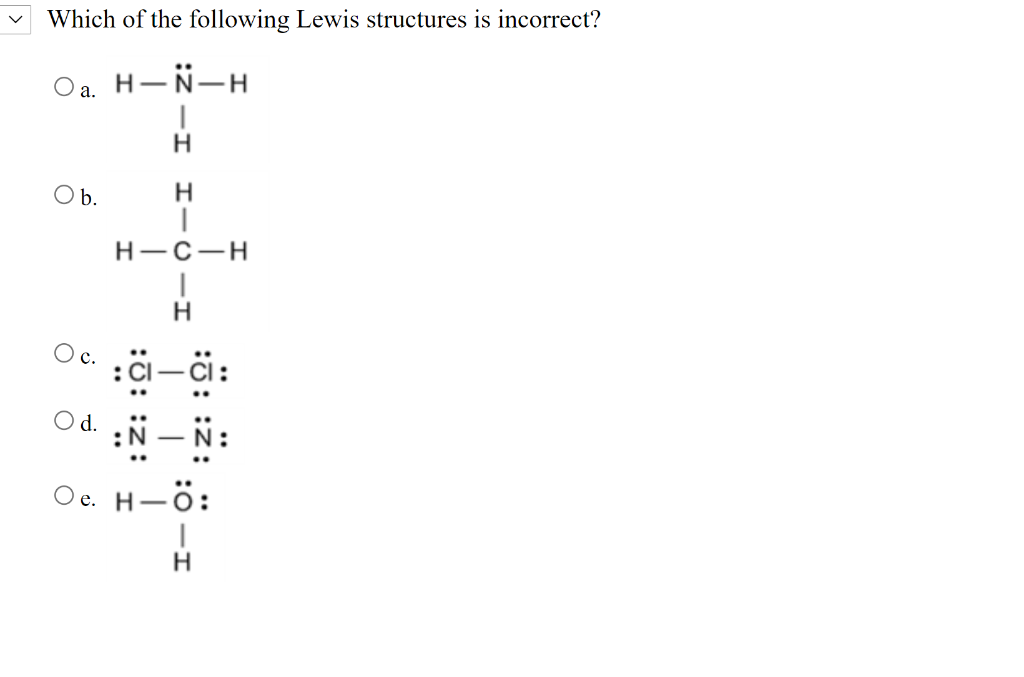
How many valence electrons does nitrogen have?
5
Identify the conjugate acid-base pairs in the following equation:
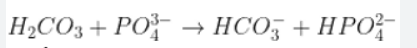

How many grams of oxygen gas are contained in 300 mL at STP?
0.428 g O2
How much heat is released when 100 g of CuBr is reacted with Ni?
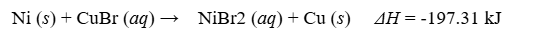
68.77 kJ
A laser emits light at 600 nm. What is the frequency of this radiation?
f = c/
f = 3.0x108 / 6.0 x 10-7
f = 5.0 x 1014 Hz
What are the formal charges on each element?
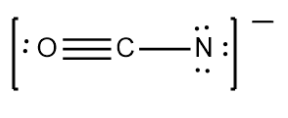
O = +1
C = 0
N = -2
What is the [OH-] in a solution with a pH of 3.5?
3.16 x 10-11 M
At constant temperature, 10 L of CO2 at 0.65 atm is compressed to 4.3 L. What is the final pressure?
1.51 atm
356 Joules of heat is required to change the temperature of 10 grams of a substance from 25 0C to 40 0C. What is the specific heat of the substance?
2.37 J/g0C
What are the noble gas electron configuration for:
a.) aluminum ion
b.)sulfide ion
c.) iron +3 ion
a.) [Ne]
b.) [Ar]
c.) [Ar] 3d5
What is the molecular geometry of NO3-1?
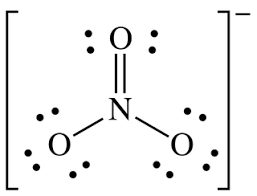
trigonal planar
What is the pH of a 25 mL of a 0.15 M solution of HCl?
0.824
A mixture of gases has a total pressure of 2.45 atm. The mixture contains 10 g of oxygen, 10 g of hydrogen, and 10 grams of carbon dioxide. What is the partial pressure of hydrogen?
2.21 atm
Determine the ∆H of the reaction using the given information.
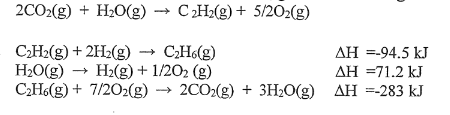
235.1 kJ
Answer the following questions:
a.) What energy level contains 9 orbitals?
b.) Which of the following atoms is diamagnetic?
Na - Ni - Cl - No - N
c.) What element has the ground state electron configuration of 1s22s22p63s23p64s13d10
a.) 3rd energy level
b.) No
c.) Cu
D.
Which of the following statements is/are true for a 0.10 M solution of a strong acid? Select all that apply.
a.) pH = 1
b.) [H+] > [A-]
c.) [H+] = [A-]
d.) [H+] < [A-]
e.) pH < 1.0
f.) pH > 1.0
a.) pH = 1
c.) [H+] = [A-]
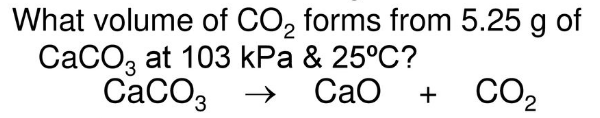
1.26 L
75 mL of water at 20 0C is combined with 189 mL of water at 67 0C. What is the final temperature of the water?
53.65 0C
A laser operates at 5.0 x 103 nm. What is the energy of 1 mole of photons with this wavelength?
23, 933.11 J
Which of the following molecules are polar? Select all that apply.
CO2 - CH4 - H2O - NH3 - SF6
NH3 and H2O
Calculate the amount of heat required to change 10 g of ice at -5 0C to water at 10 0C.
q = (10)(2.09) (5) = 104.5 J
q = (10)(333) = 3,330 J
q = (10)(4.184)(10) = 418.4 J
qtotal = 3852.9 J