Reasoning
What is the largest reservoir of nitrogen on the planet?
A) oceans
B) plants
C) soil
D) atmosphere
E) sediments, including fossils
D) atmosphere
In a reaction between Mg and Ar, which of the following is most likely to occur?
A) no reaction would occur
B) a precipitate would form
C) a gas would be produced
D) a color change would occur
E) an aqueous solution would form
A) no reaction would occur
Which of the following statements best describes the structural relationship between cis-1,2-dibromocyclopropane and trans-1,2-dibromocyclopropane?
A) The two compounds are mirror images of one another
B) The two compounds can be separated by ordinary physical chemical separation methods
C) The two compounds have the same melting points
D) All of the above
B) The two compounds can be separated by ordinary physical chemical separation methods
Which one of the following properties applies to alkaline earth metals?
A) Dull
B) Form 2+ cations
C) High density
D) Low boiling point
E) Low melting point
B) Form 2+ cations
What is the ratio of the number of composite integers to the number of prime integers in the intervals 1-10?
A) 5:4
B) 3:2
C) 1:1
D) 4:5
E) 2:3
D) 4:5
The human respiratory rate is regulated by chemoreceptors that monitor which of the following?
A) oxygen
B) methane
C) pH levels
D) hemoglobin
E) proton levels
C) pH levels
Which piece of laboratory glassware is best used to measure 1.000 L of solution accurately?
A) Beaker
B) Buret
C) Volumetric pipette
D) Measuring cylinder
E) Volumetric flask
E) Volumetric flask
Which labeled proton in the following molecule is most acidic?
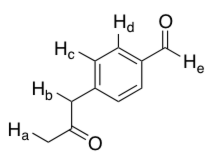
A) a
B) b
C) c
D) d
E) e
B) b
Osteoporosis is a disease that causes excessive bone resorption in individuals suffering from it. This excessive bone resorption can lead to
A) Decreased calcium levels
B) Increased phosphorus levels
C) Increased parathyroid hormone levels
D) Decreased calcitonin levels
B) Increased phosphorus levels
A 3000-gallon tank has a valve at the top that takes in gasoline at a rate of 50 gallons per minute, and a spigot at the bottom that empties it at a rate of 20 gallons per minute. If the tank is initially empty, and the valve and the spigot are open, how long will it take to fill half the tank?
A) 30 minutes
B) 45 minutes
C) 50 minutes
D) 55 minutes
E) 65 minutes
C) 50 minutes
In a population of mice, the coat color varies between dark fun (dominant) and light fur (recessive). The frequency of the homozygous recessive genotype is 16%. Assuming the population is in Hardy-Weinberg equilibrium, which of the following would be the allele frequency of the dominant (dark fur) allele in the mice population?
A) 0.16
B) 0.4
C) 0.48
D) 0.6
E) 0.84
D) 0.6
What is the bond angle that exists within a molecule of SO3?
A) 90
B) 104.5
C) 107
D) 109.5
E) 120
Which one of the following reactions of alkenes does the Anti-Markovnikov’s rule apply instead of Markovnikov’s rule?
A) Hydration
B) Oxymercuration-demercuration
C) Hydroboration-oxidation
D) Addition of HCl
E) Addition of Br2
C. Hydroboration-oxidation
Which of the following pairs are typically found in alkaline buffer solutions?
A) Weak base and its conjugate acid
B) Weak acid and its conjugate base
C) Strong base and its conjugate acid
D) Strong acid and its conjugate base
A) Weak base and its conjugate acid
A committee of 4 people is to be selected from a group of 9. If the selection is made randomly, how many different groups can be made?
A) 24
B) 126
C) 3024
D) 6561
E) 15120
B) 126
What is the correct order of stages in pre-embryonic development?
A) Cleavage, zygote, blastocyst, morula, implantation
B) Cleavage, zygote, morula, blastocyst, implantation
C) Zygote, cleavage, morula, blastocyst, implantation
D) Zygote, cleavage, blastocyst, morula, implantation
E) Zygote, morula, blastocyst, implantation, cleavage
C) Zygote, cleavage, morula, blastocyst, implantation
In which situation is water acting solely as a Bronsted-Lowry base?
A. NH3 + H2O -> NH4+ + OH-
B. NaCl(s) + H2O -> Na+(aq) + Cl-(aq)
C. HNO3 + H2O -> NO3- + H3O+
D. CuSO4(s) + 5 H2O -> CuSO4 . 5H2O(s)
E. 2 H2O -> H3O+ + OH-
C. HNO3 + H2O -> NO3– + H3O+
Which of the following compounds would be considered aromatic?
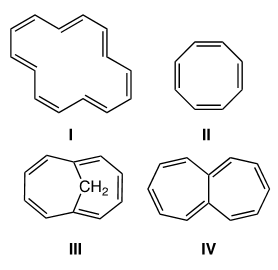
A) I and IV
B) II and III
C) II and IV
D) I and III
E) all of the above
D) I and III
Which hormone sees a significant increase immediately during ovulation?
A) FSH
B) LH
C) Estrogen
D) Progesterone
E) hCG
B) LH
The probability that Joe wins his first boxing match is 0.47. The probability that Joe wins his second boxing match is 0.85. What is the probability that Joe wins at least one of these two matches?
A) 0.50
B) 0.61
C) 0.75
D) 0.85
E) 0.92
E) 0.92
The group of organisms that are amoeboid and exhibit a slug stage are:
A) Rhizopods
B) Bryophytes
C) Cellular slime molds
D) Dinoflagellates
E) Zoomastigotes
C) Cellular slime molds
How many electrons are involved in the following basic redox reaction?
4 Zn + NO3- + 7 OH- + 6 H2O ---> 4 Zn(OH)42- + NH3
A) 2
B) 4
C) 6
D) 8
E) 10
D) 8
Which reagents would you use to perform the following reaction in high yield?
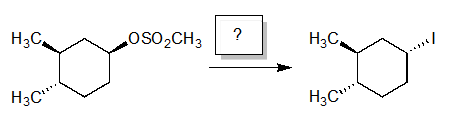
A) NaI/acetone
B) I2/hν
C) Dilute HI/H2O
D) CH3MgI/Et2O
E) LiI/H2O
A) NaI/acetone
You are the chosen one.
500 free points
Quantity A: The numerical value for the internal angle of a regular 14-gon.
Quantity B: The numerical value for the area of a circle with circumference 16 pi.
A) Quantity A is greater
B) Quantity B is greater
C) The quantities are equal
D) The relationship cannot be determined from the information given
B) Quantity B is greater