The number of mL of water in a vessel after 3.09 mL of water is added to 1.08 L of water.
(1 L = 1000 mL
What is 1.083x103 mL?
Why???
1.08 L = 1080 mL
3.09 mL
+ 1080. mL
____________
= 1083.09 mL
+/- s.f. rules: fewest digits past the decimal
=1083 mL = 1.083x103 mL
The periodic trend that refers to an element's affinity for electrons.
What is electronegativity?
What is nuclear fusion?
The formula and the name of the compound consisting of nitrogen and calcium.
What is Ca3N2, calcium nitride?
The rule that we use to determine the number of bonds the four main covalently bonding elements have.
What is HONC 1234?
The number of significant figures in the answer to the problem 0.00560201110 * 9007.92000468.
What is 9?
The difference between homogenous and heterogenous mixtures.
What is the ability to see a mixture's separate components?
The remaining daughter isotope if Lead-208 goes through alpha decay twice and beta decay once.
What is Gold-200?
The name of spectrum outlining all the different types of light.
What is the electromagnetic spectrum?
The error(s) in the following structural formula: 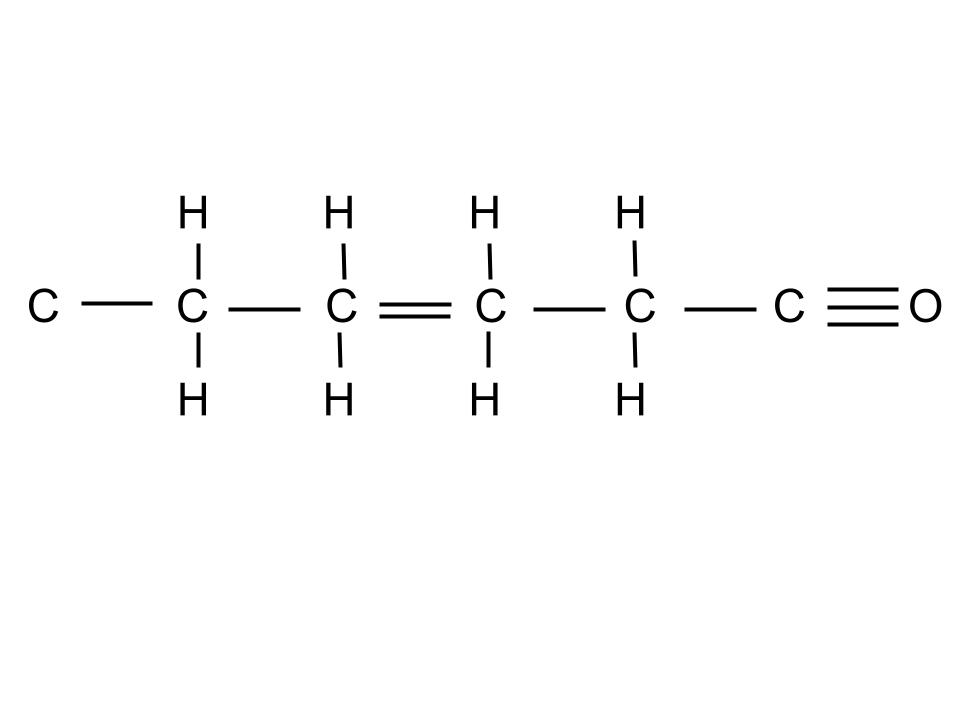
What is: 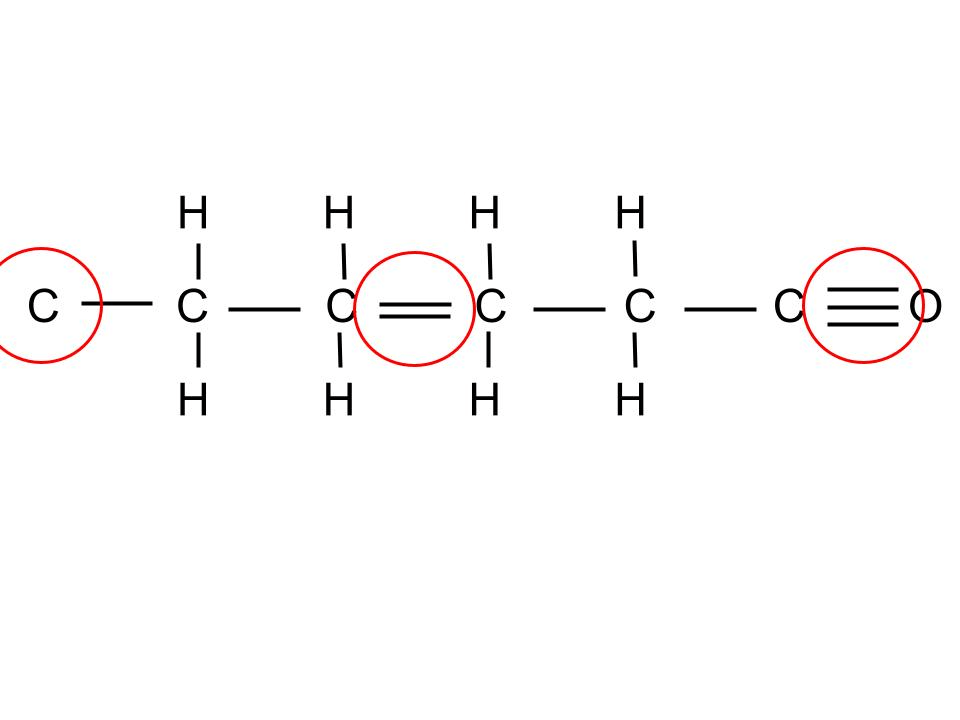
All of the emergency safety equipment in this classroom.
What are the safety shower, eyewash station, fire extinguishers, fire blanket, first aid kit, and evacuation backpack.
What is 77 neutrons?
The process of slowing down or stopping neutrons in a nuclear power cell.
What is neutron moderation or neutron absorption?
The frequency of light that coincides with a wavelength of 423 nm.
c=λν
ε=hν
c=2.998x108 m/s
1 nm =1x10−9 m
h=6.626x10−34 Jᐧs
What is 7.09x1014 s-1?
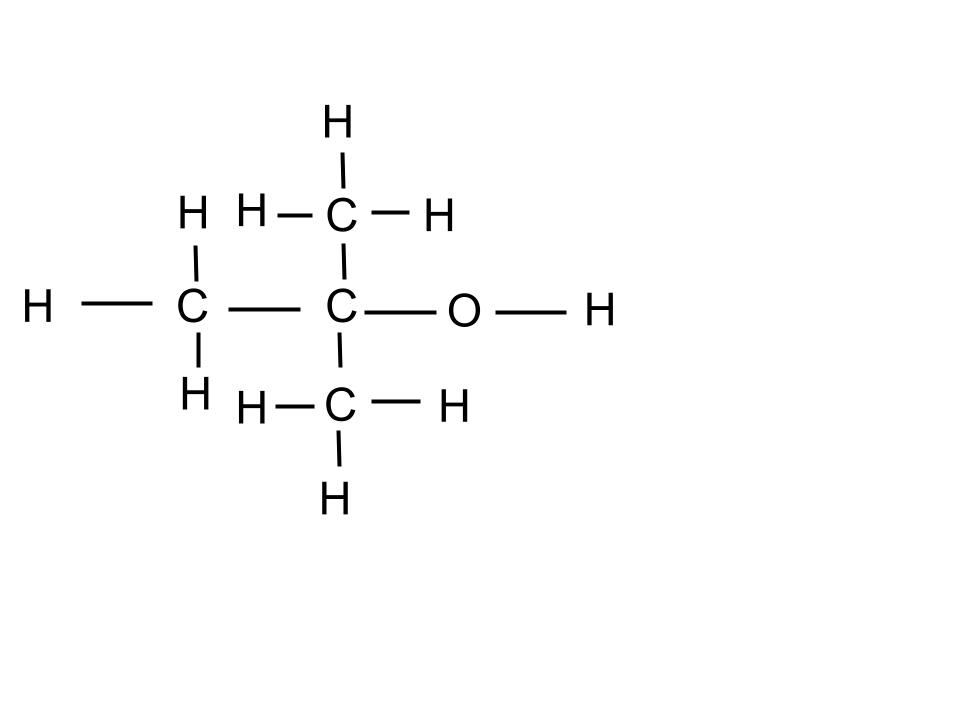
2 different isomers of C4H10O.
What are: 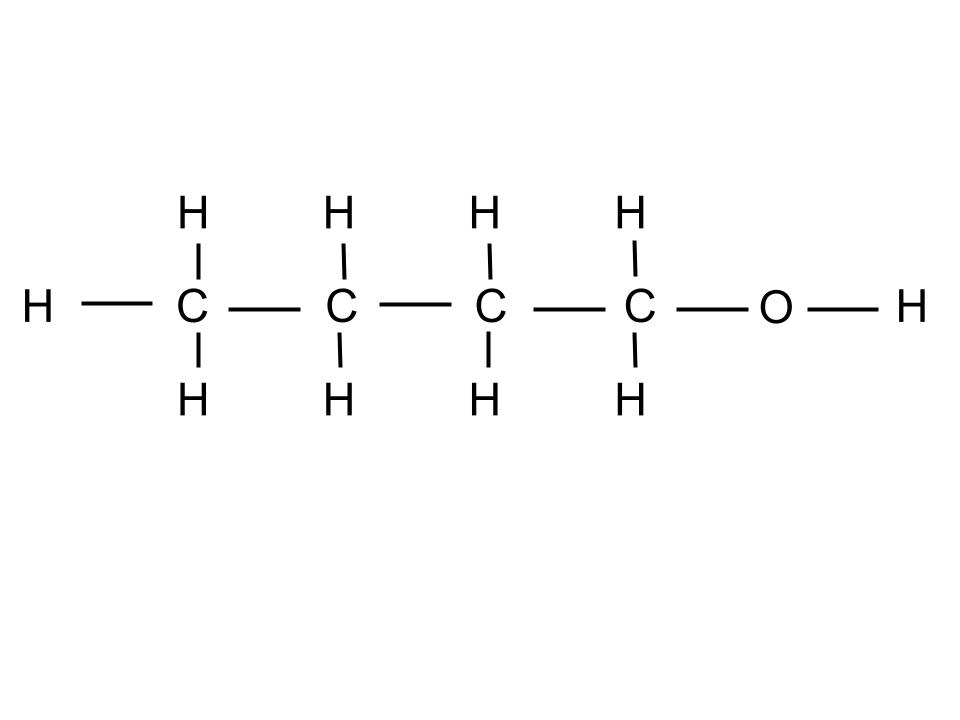
The volume in L of liquid when 0.59710 L are removed from a 780.23 mL container, combined with an additional 23.09 mL, then added to a container with 901 mL inside.
What is 1.107 L?
A drawing of the periodic table containing 4 different periodic trends.
What is: 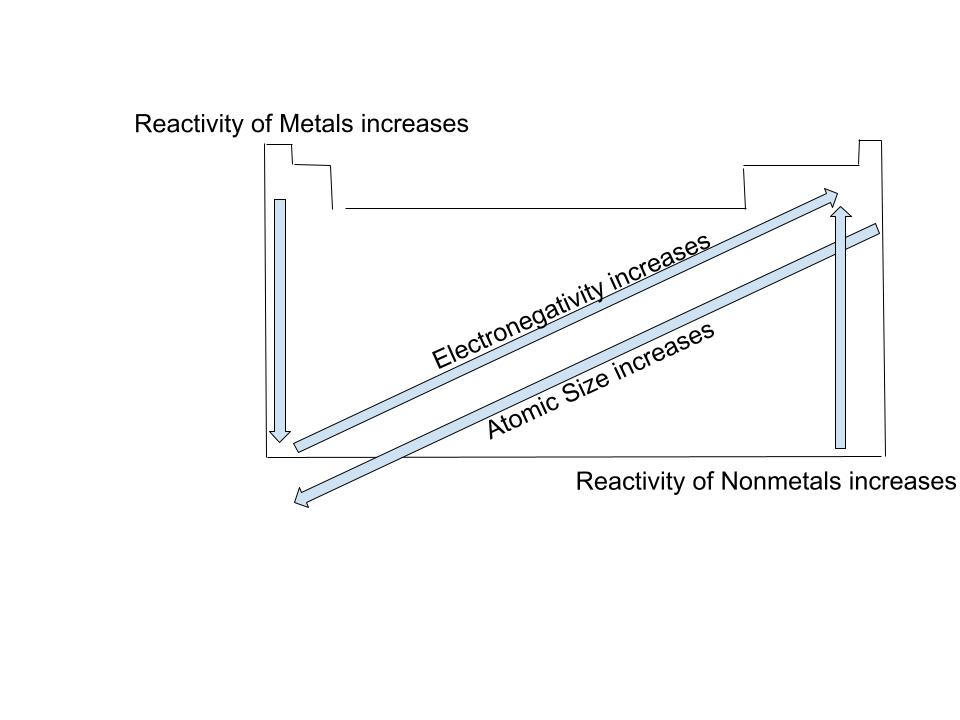 ?
?
The amount of time it takes for a 10.0 g Carbon-14 sample to decay to 1.25 g.
carbon-14 half life: 5730 years
What is 17,190 years?
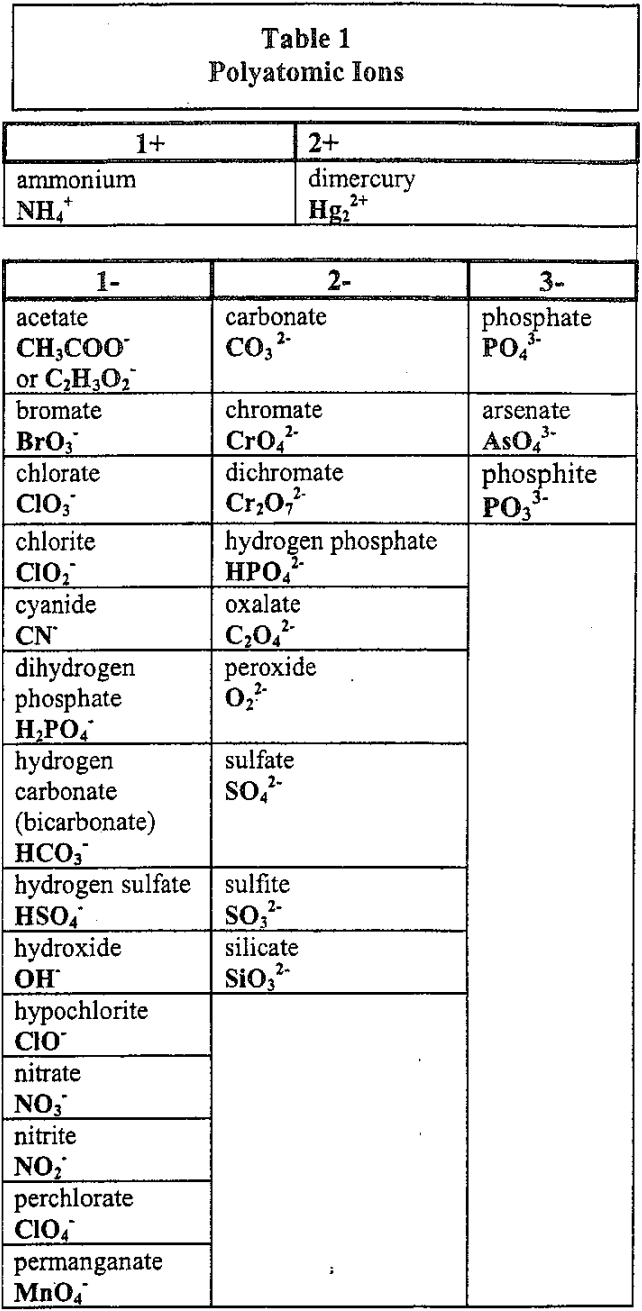
10 polyatomic ion formulas with names.
What are: (any 10 of the following)
The names of 10 different covalently bonded molecules.
What are:
(answers vary)
The mass in g of an object with a density of 6.7 kg/L that displaces water in a graduated cylinder from 56.90 mL to 77.11 mL.
1000 mL = 1 L
1000 g = 1 kg
What is 1.4x102 g?
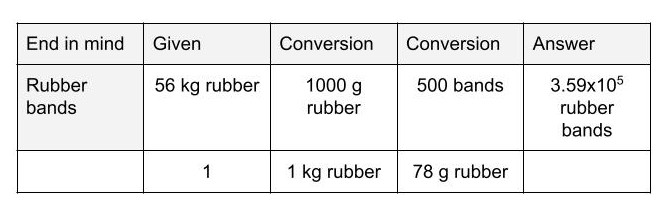
The number of rubber bands that can be made out of 56.0 kg of rubber if 78.0 g of rubber produces 500. bands (answer must contain correct dimensional analysis work, correct significant figures, scientific notation, and units).
1 kg = 1000 g
What is 3.59x105 rubber bands?
A perfect example equation for nuclear fusion.
What is 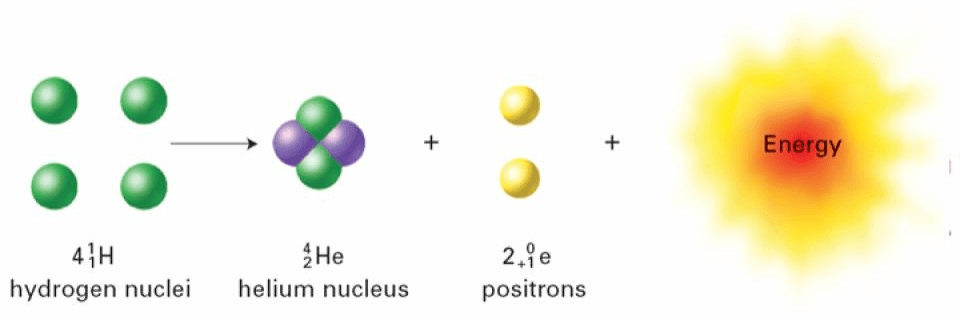
?
The full electron configuration for Oganesson.
What is: 1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p67s25f146d107p6 ?
What are:
- alkyl halide (smell depends)
- alcohol (camphor)
- ether (pungent/sweet)
- amine (fishy)
- ketone (minty)
- aldehyde (spicy)
- carboxylic acid (putrid)
- ester (sweet)