The density of a 67 g rock that displaces 74 mL of water in a graduated cylinder.
What is 9.1 g/mL?
The name of the element with 1 valence electron that is NOT a metal.
What is hydrogen?
The type of reaction that involves rearranging atoms into new substances.
What is a chemical reaction?
A potential scenario that would require using the chemical shower.
What would be getting chemicals on your clothes, face, or eyes?
The weight of this semester's final exam according to the syllabus.
What is 10% of the grade?
What is 4 half lives?
The name and formula of the compound made from Zr2+ and permanganate.
What is zirconium (II) permanganate, Zr(MnO4)2?
The type of change that occurs and state of matter that results when lemonade powder is mixed into liquid water.
What is a physical change into an aqueous state?
The result if the cooling failsafe in a nuclear reactor malfunctions.
What is a nuclear core meltdown?
The names and structural formulas for the two functional groups that produce a sweet smell.
What are: ?
?
A potential full structural formula of nonyne.
What is:

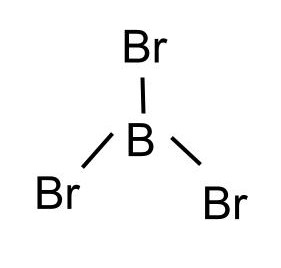
The name, structural formula, and shape of the molecule made of three bromine atoms and a boron atom.
What is the trigonal planar molecule, boron tribromide, BBr3?
The type of reaction (and it's name) of the process of an electron bombarding a nucleus and producing the emission of a neutrino.
What is the nuclear reaction called electron capture?
What is molecular covalent?
Two different isomers for the formula C6H12O6.
What are [answers vary]?
The number of kg of rubber needed to make shoes for 56.0 days if it takes 3.00 days to make 777 shoes and 78.8 g of rubber per shoe.
1000 g = 1 kg
What is 1.14x103 kg of rubber?
The name, formula, and Lewis Dot formula of a polyatomic ion containing phosphorus and oxygen.
What is phosphate, PO43- 
OR
What is phosphite, PO33-

What is 19778Pt --> 19779Au + 0-1e + energy ?
The correct emergency response in case of a seismic natural disaster during a lab.
What is leaving everything where it is, crouching under a table or counter, and protecting your head?
Two reasons why your chemistry teacher is making you learn functional groups for the upcoming exam.
What are 1) connections to organic chemistry for biology-related fields, 2) further understanding about one of your main evolutionary senses, or 3) [other reason acceptable to your teacher]
The color of light produced with 3.36x10-19 J of energy.
I hope you know the light formulas!
speed of light = 2.998x108 m
1 nm = 1x10-9 m
planks constant = 6.626x10-34 J*s
What is orange light?
The name, molecular formula, empirical formula, and functional group of the following molecules.
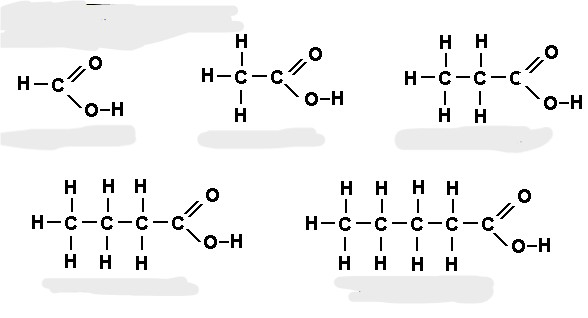
What is:
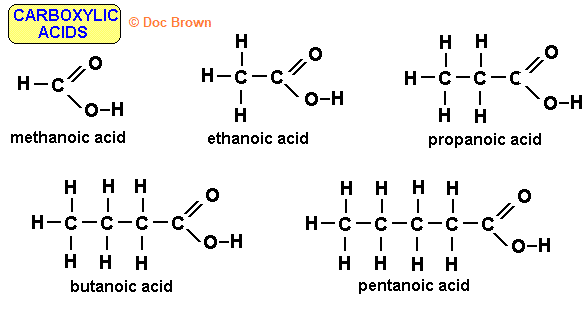
methanoic acid: CH2O2
ethanoic acid: C2H4O2 ; CH2O
propanoic acid: C3H6O2
butanoic acid: C4H8O2 ; C2H4O
pentanoic acid: C5H10O2
Full equation below written entirely in words.
[HINT: "+" means "and" ; "-->" means "makes" or "react to make"]
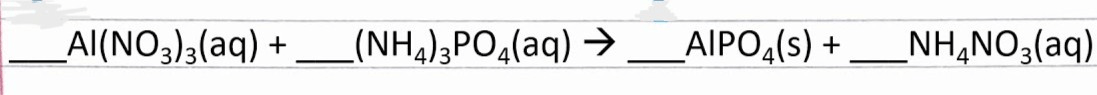
What is 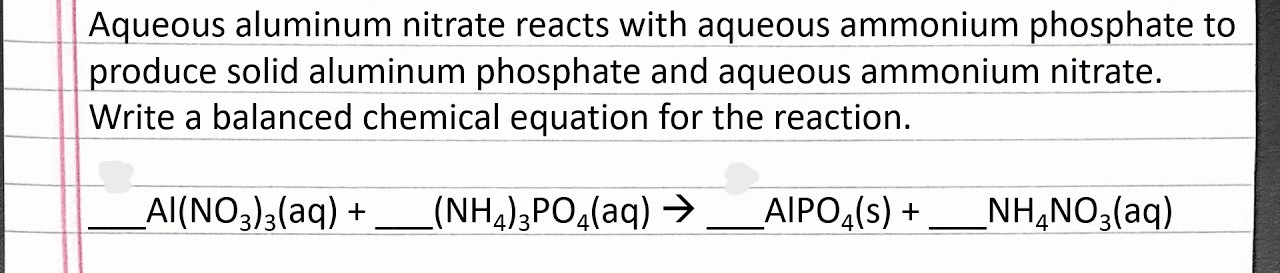
What is 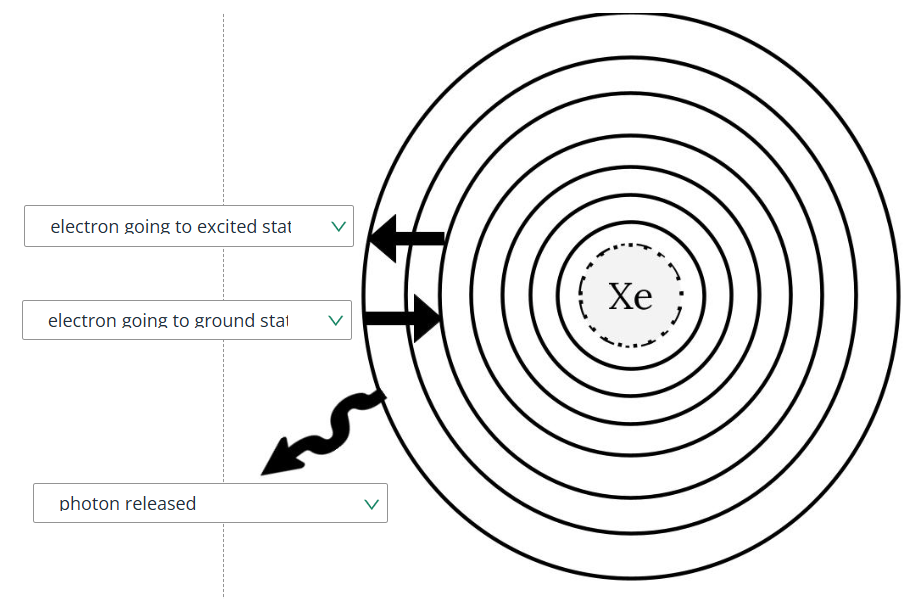 ?
?
What is technetium?