All matter has _____ and _____.
Mass and Volume
We measure mass using a _______ and the units are ______.
Scale
Grams
The elevator on the ____ has more density because ____.
Right
It has more people in the same area (Or the people are more packed together)
The density of water is ____.
1 g/mL
Name the states of matter from coldest to hottest.
Solid
Liquid
Gas
A ____ change will make a new substance, but a ____ change will not make a new substance.
Chemical
Physical
The amount of matter in an object
Mass
We measure volume using a ______ and the units are ______.
Graduated Cylinder
milliLiters (mL)
The _____ has a higher density because ______.
The baseball has a higher density because it has more mass in the same volume. (Or the particles are more packed together)
Objects with a density more than 1 g/mL will _____, but objects with a density less than 1 g/mL will _____.
Sink
Float
We can change the state of matter by changing the ______.
Temperature
What question can we ask ourselves to decide between a physical and chemical change?
"Did I make a new substance?"
The amount of space an object takes up
Volume
We measure density using a _____ and the units are _____.
g/mL
These boxes have the same ______, but Box B has more _____.
Same Volume
More Mass and Density
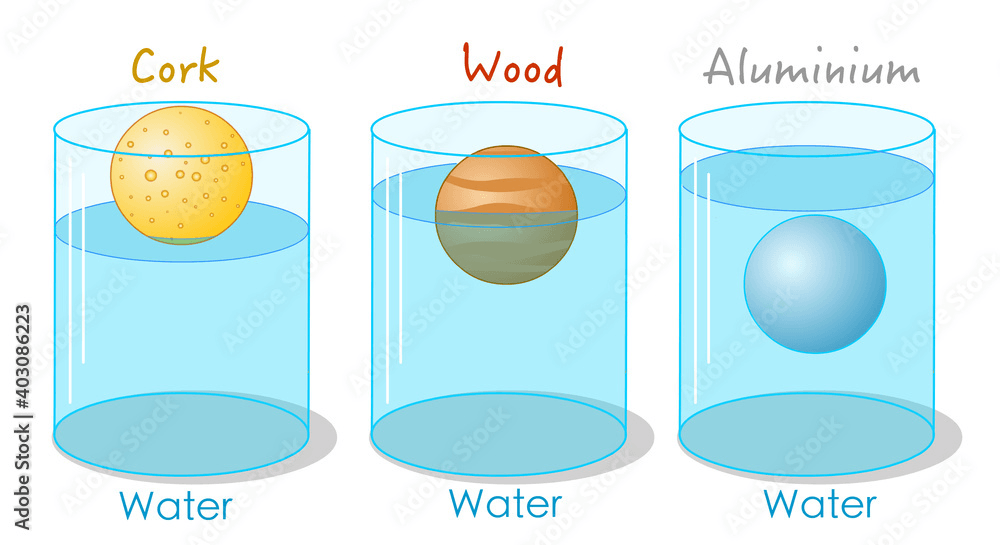
What can we infer about the density of:
- Cork
- Wood
- Aluminum
- Cork - Less than 1 g/mL
- Wood - Equal to 1 g/mL
- Aluminum - More than 1 g/mL
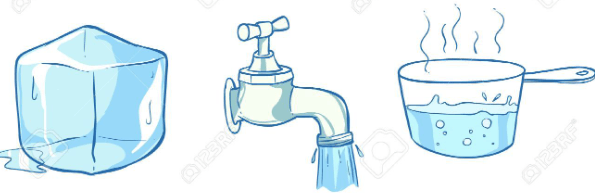
Describe the states of matter you observe.
The ice cube is a solid.
The water in the faucet is a liquid.
The water in the pot is a gas.
Baking a cake is a ___ change because ____.
Slicing a cake is a ___ change because ____.
Chemical - we started with ingredients and ended with cake, so we made a new substance
Physical - we started with a big cake and ended with smaller cake, but we did not make a new substance
Density is the amount of ______ in a specified ______.
Mass
Volume
Measurements of mass using a scale or volume using a graduated cylinder are described as ____ observations because ______.
If we did not have measurement tools, we would need to use ____ observations, which have _______.
Quantitative; they use numbers
Qualitative; they use adjectives
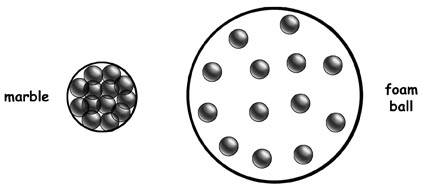 These balls have the same ______, but the marble has _______ and ______.
These balls have the same ______, but the marble has _______ and ______.
Same Mass
Marble has less volume and more density
If a log floats in water, what do I know about each piece of log that I chop off?
It will also float because density does not change.
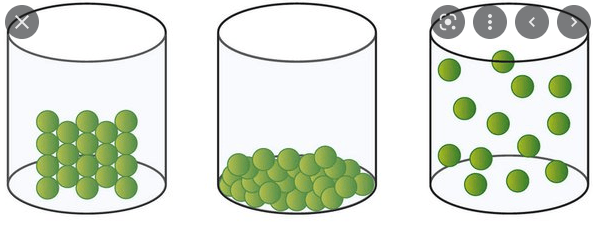
What states of matter do you observe? How do you know?
The first beaker is a solid because the particles are packed close together.
The middle beaker is a liquid because the particles are not packed close together or spread apart.
The final beaker is a gas because the particles are very spread apart.
Give an example of a physical and chemical change involving a piece of wood.
Physical
- Chop the wood, break the wood, hammer a nail into the wood
Chemical
- Burn the wood

Peter needs to transport 1000 balloons to a party across town.
Should he choose a company that charges by mass or volume?
"I think he should choose the company that charges by ____ because ____."
During COVID, the requirement of social distancing ___ the density of locations because _____.
Decreased
People had to spread out
These boxes have the same _____, but the blue box has _____ and _____.
Same Mass
More volume and less density
You have three liquids:
- Water
- Blood (1.6 g/mL)
- Alcohol (0.8 g/mL)
You pour them into a cup and leave it overnight. What do you expected in the morning?
- The Blood will be on the bottom
- The water will be in the middle
- The alcohol will be on top
What happens to the density of materials as their temperature increases?
As temperature increases, density decreases because the particles spread out.
Give an example of a physical change and a chemical change that happens in digestion.
Physical - Teeth break food into bits; stomach muscles break bolus into bits
Chemical - Saliva turns food into bolus; stomach acids turn bolus into nutrients
