Mass measures how much _________ is in an object.
Matter
Explain how we measure the volume of a cube or rectangular solid.
A formula- V = L x W x H
If a liquid has a mass of 100 grams and a volume of 150 mL, what is its density?
Density= Mass / Volume
0.67 g/mL
(Density = 100g /150 mL)
1000 mL of a liquid has a mass of 740 grams. What is the liquid?
(Density = Mass /Volume)
Gasoline
740 g / 1000 mL = 0.74 g/mL
Mass and Weight are similar but not the same. How are they related? As mass increases, weight _______.
Increases!
Explain how mass is measured
A scale
What is one of the units of measurements that we use for volume?
Cm3 or mL
25 cm3 of a substance has a mass of 87.5 grams. What is its density?
(Density = Mass/Volume)
3.5 g/cm3
Density = 87.5 g/25 cm3
91 grams of a substance has a volume of 7 mL. What is the substance?
(Density = Mass / Volume)
Mercury
91 grams / 7 mL = 13 g/mL
What is the formula used for calculating the density of an object?
Density = Mass / Volume
What unit of measurement is used for mass?
Grams or Kilograms
What is the volume of this shape?
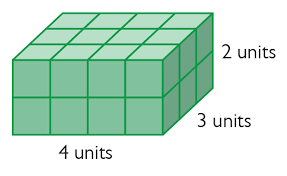
V = L x W x H
V = 4 x 3 x 2 = 24 units
495 grams of a metal occupies a volume of 55 cm3. What is the metal's density?
(Density = Mass/Volume)
9 g/cm3
Density = 495 g/55 cm3
4000 grams of a substance occupies 500 cm3 of space. What is the substance?
(Density = Mass / Volume)
Brass
4000 grams / 500 cm3 = 8 g/cm3
Give an example of a unit of measurement we use for density.
False- Weight is affected by gravity, but mass always stays the same.
What is the volume of the 2 marbles?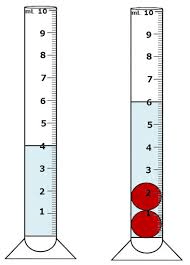
6 mL - 4 mL = 2 mL
(Water displacement method)
What volume of aluminum would have a mass of 54 g?
Volume = Mass / Density
20 cm3
(Volume = 54 g / 2.7g/cm3)
12 mL of a liquid has a mass of 13.32 grams. What is the liquid?
(Density = Mass / Volume)
Antifreeze
Density = 13.32 grams / 12 mL = 1.11 g/mL
Which would float in water: Object A with a density of 1.2 g/ml or object B with a density of 0.5 g/ml?
Object B- the density of Object B is less than the density of water!
Use your Table of Densities to determine which would have greater mass- 1 cm3 of copper or 1 cm3 of steel?
1 cm3 of copper- the density of copper is higher!
Use your Table of Densities to determine which material would have the greatest volume- 50 grams oak wood or 50 grams of aluminum?
50 grams of oak wood - the density of oak wood is lower, meaning it would take more volume to equal the same mass as the aluminum
What mass of salt water would have a volume of 1200 ml?
(Mass = Volume x Density)
1,440 grams
(Mass = 1200 mL x 1.2 g/mL)
8200 cm3 of a gas has a mass of 10.66 grams. What is the gas?
(Density = Mass / Volume)
Carbon dioxide
10.66 grams / 8200 cm3 = 0.0013 g/cm3
If a metal block has a density of 20 g/cm3, what would the block's density be if it was cut in half?
20 g/cm3
The density would stay the same! Density does NOT depend upon size.