Most of the mass of an atom is here.
What is the nucleus?
Has atomic number 2.
What is helium?
An atom's ability to attract a valence electron from another atom.
What is electronegativity?
The mass of this atom
12 -1
C
6
What is the number 12?
The element group that you use for Noble Gas Configuration.
What are Noble Gases?
Positively charged particle in an atom.
What is a proton?
Electrons in the outermost shell, or energy level, of an atom
What is a valence electron?
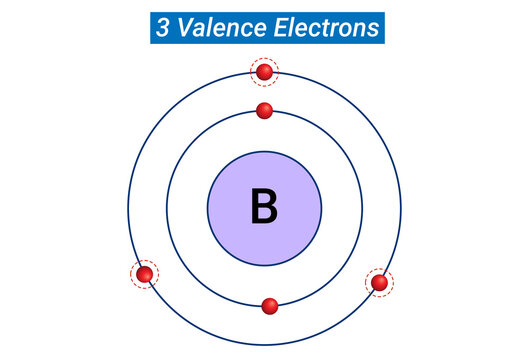
the energy required to remove a valence electron from an atom.
What is ionization energy?
The charge of Fluorine (F).
18 +2
F
9
What is the number 2?
The Full Electron Configuration of Potassium (K).

What is 1s^2, 2s^2, 2p^6, 3s^2, 3p^6, 4s^1?
Protons and Neutrons
What are particles found in the nucleus of an atom?

The only nonmetal on the left side of the periodic table.
What is hydrogen?

It's the distance from an atom's nucleus to the outermost electrons.
What is Atomic Radius?
The atomic number of Selenium (Se).

What is the number 34?
The Noble Gas Configuration of Rubidium (Rb).

What is [Kr] 5s^1?
Negatively charged particles found in atoms.
What are electrons?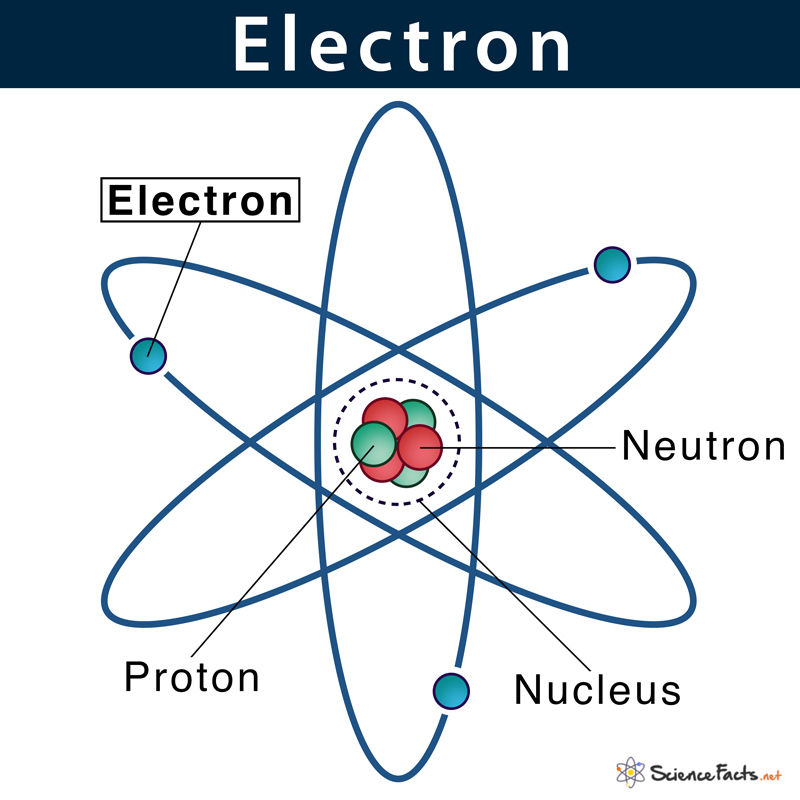
The four primary elements in living things.
What is carbon, hydrogen, oxygen, and nitrogen?
The element that has the most atomic radius. Potassium (K) or Sulfur (S).
The number of electrons in this picture.
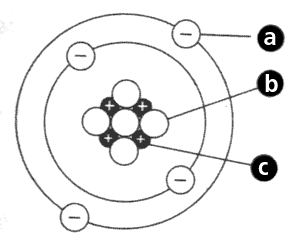
What is the number 4?
The Full Electron Configuration of Oxygen (O).

What is 1s^2, 2s^2, 2p^4?
An atom that has either lost or gained an electron.
What is an ion?
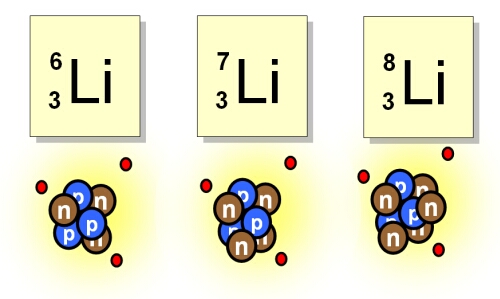
Elements with varying numbers of neutrons.
What are isotopes?
The element that has more ionization energy. Barium (Ba) or Tin (Sn).
The number of protons in this picture.
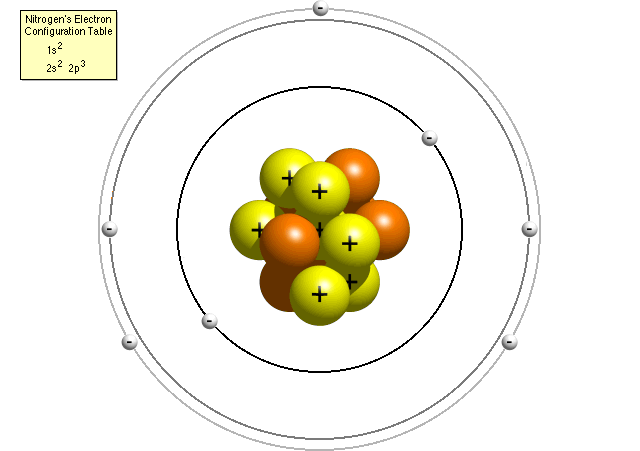
What is the number 7?
The Noble Gas Configuration of Arsenic (As).

What is [Ar] 4s^2 3d^10 4p^3?
