Aluminium is extracted from its oxide by electrolysis. The oxide is dissolved in ___1____ cryolite and aluminium is deposited in the ____2______.
C
The diagram shows the electrolysis of concentrated aqueous sodium chloride. What is the colour of the indicator at each electrode after 5 minutes?
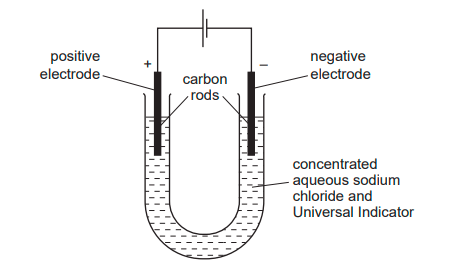
Cathode is blue/purple, Anode is colourless due to chlorine´s bleaching effect.
Which of these elements could be formed at the anode when a molten salt is electrolysed?
Copper
Iodine
Lithium
Strontium
Iodine
The diagram shows apparatus for plating a spoon with silver.
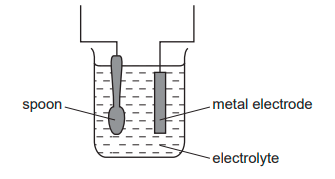
Which statement is not correct?
A) Silver would stick to the spoon because it is a very reactive metal
B) The electrolyte would be a silver salt dissolved in water
C) The metal electrode would be made from silver
D) The spoon would be connected to the negative of the power supply
A since silver is low down in the reactivity series.
Metals could be extracted from their molten chlorides using electrolysis. Which substances are formed at each electrode?
Anode - Chlorine
Cathode - Metal
When concentrated sodium chloride solution is electrolysed, elements X and Y are formed. What are X and Y?
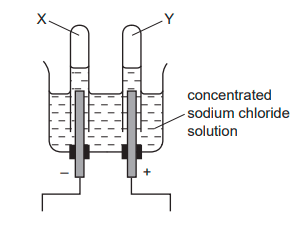
X = Hydrogen
Y = Chlorine
The diagram shows an incomplete circuit.
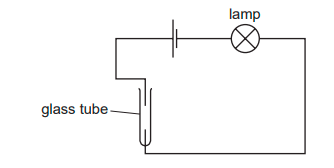
Which substance causes the lamp to light when added to the glass tube? A aqueous sodium chloride B aqueous sugar C solid sodium chloride D solid sugar
A
Which metal could not be used for electroplating by using an aqueous solution?
A chromium
B copper
C silver
D sodium
Sodium
The diagram shows how aluminium is manufactured by electrolysis. What are the anode and the cathode made of?
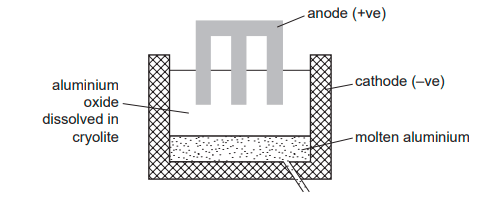
Graphite
Substance X was electrolysed in an electrolytic cell. A coloured gas was formed at the anode and a metal was formed at the cathode. What substance is X?
Aqueous Sodium chloride
Molten Lead bromide
Molten Zinc oxide
Solid Sodium chloride
Molten lead bromide
Molten lead (II) bromide is electrolysed as shown.
Which ions are discharged at each electrode?
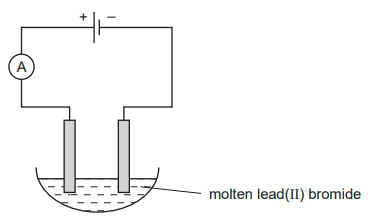
Cathode: Pb 2+
Anode: Br -
The diagram shows apparatus used in an attempt to electroplate a metal ring with copper. The experiment did not work. What change is needed in the experiment to make it work?

Reverse the connections to the battery.
Electricity from a power station passes through overhead cables to a substation and then to a school where it is used to electrolyse concentrated hydrochloric acid using inert electrodes. Which substances are used for the overhead cables and for the electrodes?
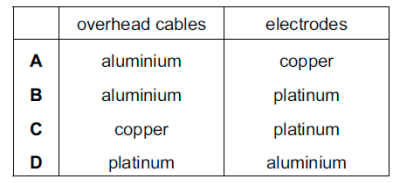
B
Metal must be a good conductor of electricity, and aluminium is more resistant than copper. Platinum is used at the electrodes since it does conduct electricity well but is a chemically unreactive metal.
Aqueous copper(II) sulfate solution is electrolysed using inert electrodes.
Copper(II) ions (Cu2+), hydrogen ions (H+ ), hydroxide ions (OH– ) and sulfate ions (SO42-) are present in the solution. To which electrodes are the ions attracted during this electrolysis?
OH– and SO42- attracted to the anode
Cu2+ and H+ attracted to the cathode
Two electrolysis experiments were carried out as shown in the diagram below. The graphite electrodes are labelled 1-4.
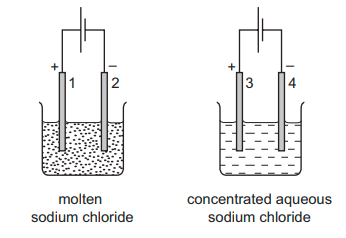
Which row describes the products at the electrodes in these experiments?
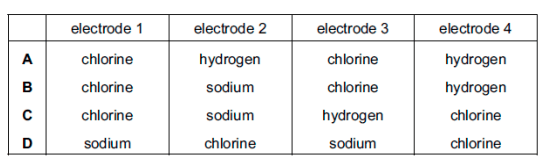
B
Molten vs aqueous solution
Winston Churchill, a British Prime Minister, had his false teeth electroplated with gold. The teeth were coated with a thin layer of carbon and were then placed in the apparatus shown.
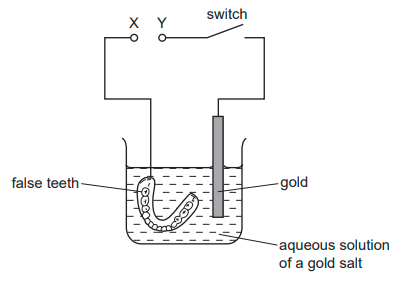 Which row is correct?
Which row is correct?
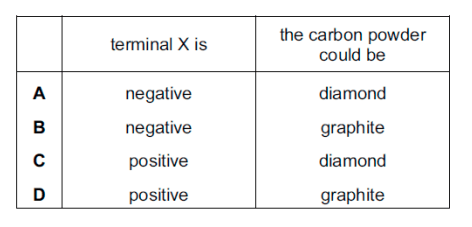
B
The metal is always the metal that is being used to coat and the object to be coated is the cathode which attracts the positive metal ions
The diagram shows the circuit for electrolysing lead(II) bromide and sodium chloride to liberate the metal. In what form are these salts electrolysed for liberating the metal?
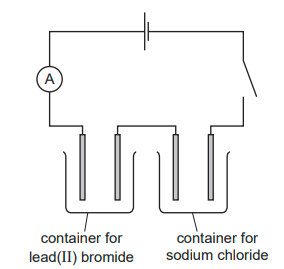
Both need to be molten
Electricity is passed through concentrated aqueous sodium chloride, as shown. What is the test for the gas formed at the positive electrode?
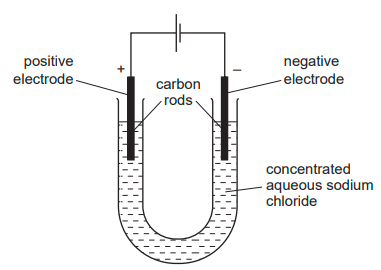
Bleaches damp litmus paper
Concentrated aqueous sodium chloride, concentrated hydrochloric acid and molten lead bromide were separately electrolysed in experiments 1, 2 and 3.
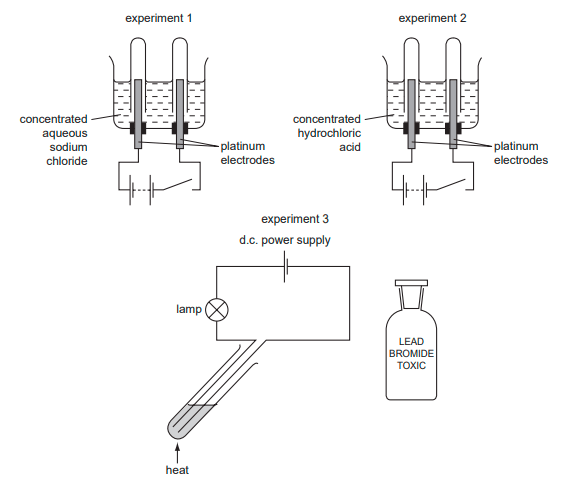
Which statement about the electrode products is correct? A Gases were given off at the anode in experiments 2 and 3 only. B Gases were given off at the cathode in experiments 1 and 2 only. C Metals were formed at the anode in experiments 1 and 3 only. D Metals were formed at the cathode in experiments 1 and 3 only.
B