the periodic table is arranged into columns known as _______ and rows known as ______
1. families or groups
2. periods
This is the electron configuration for Iron.
1s2 2s2 2p6 3s2 3p6 4s2 3d6
Lewis dot structure for the element with the following orbital notation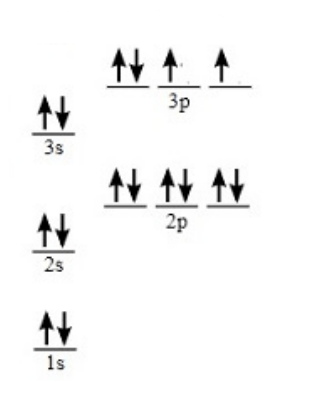

No two electrons can have the same set of quantum numbers according to _______.
Pauli Exclusion Principle
All electromagnetic waves travel at this speed
3.00 x 108 m/s
This is the equation you would use to solve for wavelength if given energy of a photon
wavelength=(planck's constant x speed of light)/(energy of a photon)
The elements along the stairstep line that separates metals and nonmetals are known as ___________
Metalloids
According to the Aufbau Principle, electrons are filled in order of
the lowest energy orbital that can receive it
Which has the greatest number of unpaired electrons?
A. 1s2 2s2 2p6 3s2
B. 1s2 2s2 2p3
C. 1s2 2s2 2p6
D. 1s2 2s2 2p4
B
What is the maximum number of electrons in an atom that have the following quantum numbers
[3, 2, -1, -1/2]
1 because of the pauli exclusion principle
This visible color has the highest energy
Violet
A wave has a wavelength of 6.68 x 10-7 m. What is the frequency?
4.49 x 1014 1/s
The Alkaline Earth Metals are found in group ____ and form ions with a _____ charge
2 or IIA
2+
The electron configuration for an element in period 6 which is considered to be the most reactive of all metals.
Cesium: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s1
Draw the Lewis structure for the element with the highest energy sublevel 4d5

Identify the quantum numbers for the 80th electron
[5, 2, +2, -1/2]
This color in the visible light range has the longest wavelength
Red
A wave has a frequency of 4.55 x 1014 Hz. What is the wavelength in nanometers? Is the wave visible?
659 nm. Yes it is visible as red light.
This element is a gas at room temperature and has one valence electron. It has this many neutrons in its most common isotope.
Zero
Write an excited state configuration for an ion that has 8 valence electrons, 16 neutrons and a charge of +15 in the nucleus
P3-: 1s2 2s2 2p6 3s2 3p5 4s1
Justify why an orbital containing two electrons must both have different spin directions
Pauli Exclusion Principle - No two electrons can have the same set of four quantum numbers.
Determine whether the following set of quantum numbers for an electron are valid
[4, 2, 3, -1/2]
The third quantum number is not valid because the d sublevel only has 5 orbitals: -2, -1, 0, 1, 2
From the diagram below, identify the wave with:
- The lowest frequency
- The shortest wavelength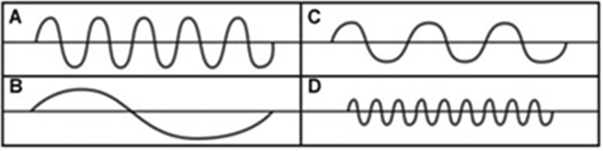
Lowest frequency - B
Shortest wavelength - D
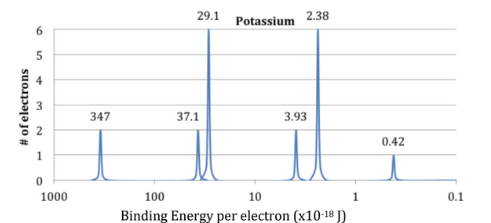 From the photoelectron spectrum above, calculate the frequency of light required to remove the valence electrons.
From the photoelectron spectrum above, calculate the frequency of light required to remove the valence electrons.
6.33 x 1014 1/s
Determine the element:
-member of the halogen family
-gas at room temperature
- in the lowest energy level containing d orbitals
Chlorine
Determine the electron configuration for an atom with the following properties:
-very reactive
-most common isotope has 74 neutrons
-highest energy electron has a down-spin
I: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p5
Identify the rule violated in each diagram or if the diagram is correct.
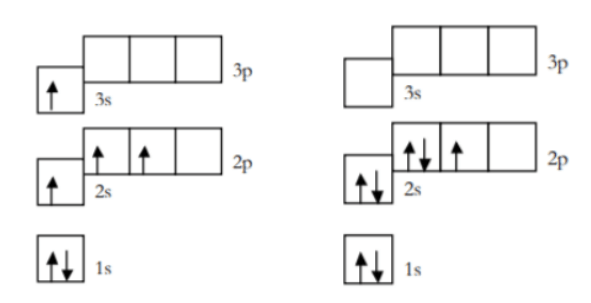
1. Aufbau Principle - An electron must occupy the lowest energy orbital that can receive it.
2. Hund's Rule- Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron, and all electrons in singly occupied orbitals must have the same spin state.
Identify the quantum numbers for the highest electron:
- located in the lowest energy having d orbitals but not in a d orbital
- has 2 valence electrons
- has a downward spin
Mg [3, 0, 0, -1/2]
Identify the element from the PES diagram below.
Aluminum
What is the number of photons of light with a wavelength of 4 nm that provide 1J of energy?
2.01 x 1016 photons
