What is Hund’s rule?
A rule stating that when filling orbitals of equal energy, electrons will occupy empty orbitals singly before pairing with other electrons
What is the full electron configuration for Li.
1s2 2s1
How many valence electrons are in the following configuration: 1s2 2s2. What element is this?
2 valence electrons, Beryllium
Write the short electron configuration for Oxygen.
[He] 2s2 2p4
Identify the blocks in the periodic table for electron configurations
Blue=s, Yellow=d, Pink=p, Green=f
What is the full electron configuration for Ar.
1s2 2s2 2p6 3s2 3p6
How many valence electrons are in the following configuration: 1s2 2s2 2p5. What element is this?
7 valence electrons, Fluorine
Write the short electron configuration for Chlorine.
[Ne] 3s2 3p5
What is the Pauli exclusion principle?
A principle stating that no more than 2 electrons can occupy an orbital and that the 2 electrons must have opposite spins
What is the full electron configuration for Ge?
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2
Write the electron configuration for the valence electrons of Bromine.
4s2 4p5
Write the short electron configuration for Calcium.
[Ar] 4s2
How many electrons can the s, p, d, and f subshells hold?
The s subshell can hold 2 electrons, the p subshell can hold 6 electrons, the d subshell can hold 10 electrons, and the f subshell can hold 14 electrons
What is the full electron configuration for Ca?
1s2 2s2 2p6 3s2 3p6 4s2
Write the electron configuration for the valence electrons of Indium.
5s2 5p1
Write the short electron configuration for Gallium.
[Ar] 4s2 3d10 4p1
What is the order of filling out orbitals? (use a diagram)
Down in a diagonal order
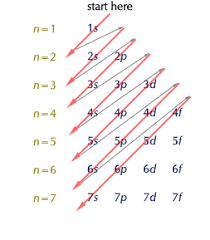
What is the full electron configuration for Sb?
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p3
Write the electron configuration for the valence electrons of Phosphorus.
3s2 3p3
Write the short electron configuration for Tin.
[Kr] 5s2 4d10 5p2
