 How many energy levels (shells) does Ca have?
How many energy levels (shells) does Ca have?
4
What type of bond forms when a metal gives electrons to a nonmetal?
Ionic Bond
Which number represents a coefficient in this example?
4H2O
4
In a chemical equation, what side of the arrow would you find the reactants?
Left side
Is this element happy?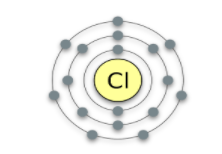
No
Covalent Bond
Which number represents a subscript for O in this example?
4H2O
1
In a chemical equation, what do you call the elements and compounds on the right side of the arrow?
The products
How many electrons will Be have on its outside shell?
2
What does this element need to do in order to be happy?
Give or Gain? How many?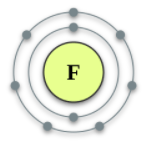
Gain 1
How many different ELEMENTS are in this chemical formula?
HNO3H
3
Hydrogen, Nitrogen, Oxygen
How many O atoms are on each side of this chemical equation?
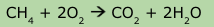
4
What do the elements in Group 18 have in common?
They are noble gases and have all energy levels/shells filled. They are all happy.
What is the chemical formula for this compound?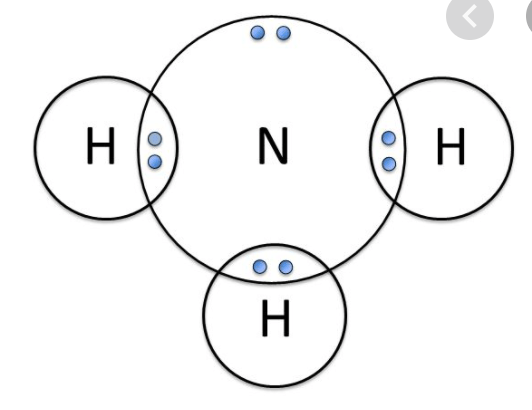
NH3
How many C atoms are in this chemical formula?
2CH4
2
Is this equation balanced? Yes or no.
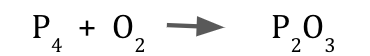
No
Reactants: P = 4, O = 2
Products: P = 2, O = 3
How many electrons can fit on each energy level/shell?
1st?
2nd?
3rd?
1st = 2
2nd = 8
3rd = 8
Which of the following best illustrates the bond for an HCl compound?
Choice A? or Choice B?
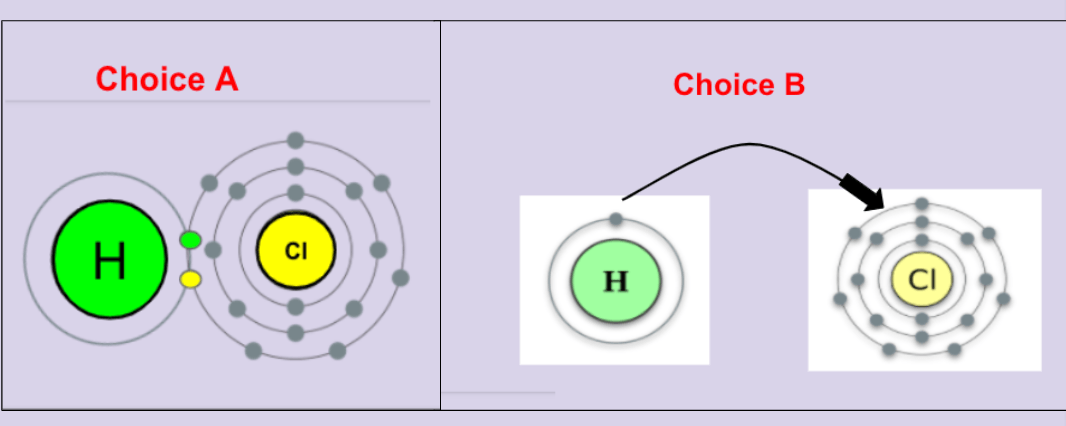
Choice A
How many Hydrogen atoms are in his formula?
5H2O
10
5 x 2 = 10
Is this equation balanced? Yes or no.
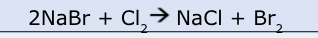
No!
Reactants: Na = 2, Cl = 2
Products: Na = 1, Cl = 1