Bond that will form with N and H
polar covalent
Polar
Bond that involves the sharing of electron pairs between atoms
Covalent
Bond that will form between N and N
nonpolar covalent
Nonpolar
PCl3
Bond formed between two ions with opposite charges
Ionic
Bond that will form between Si and Cl
polar covalent
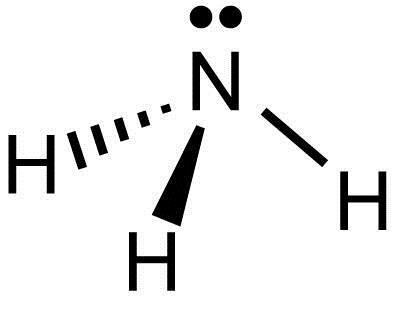
Polar
HCN

What is the mathematical product of the separation of the ends of a dipole and the magnitude of the charges?
Dipole moment
Bond that will form between Si and F
Ionic
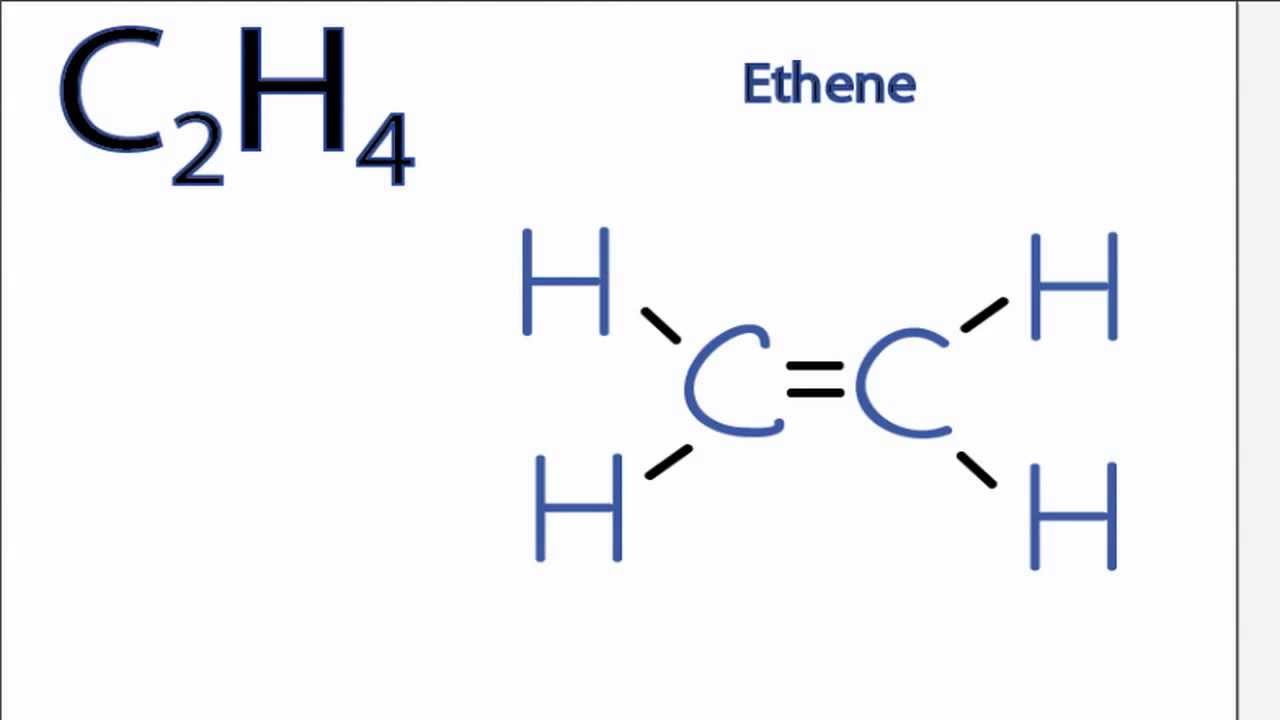
Nonpolar

Atoms that are covalently bonded, but the # of protons and electrons is not equal, so the molecule has a charge.
Bond that will form between N and K
ionic
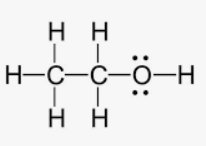
polar
What is the measure of the tendency of an atom to attract a bonding pair of electrons?
electronegativity