What does an atom lose to become positive?
an electron
What subatomic particle is used in chemical bonding (be specific)?
valence electrons
Ionic bonds form between a _____ ion and a ______ ion.
positive and negative ion
When does a covalent bond form?
when two atoms share electrons
How do the electrons move in metallic bonds?
move around freely (sea of electrons)
What does an atom have to do to become a negatively charged ion?
gain electrons
How many valence electrons are in group 16?
6
Ionic bonds form between what classes of elements?
metals and nonmetals
What class of elements does a covalent bond form between?
between nonmetals
Metallic bonds are formed by the attraction between ________ and the _________ that are around them.
positive metal ions and the sea of electrons around them
What is an ion?
a charged atom
Based on the number of valence electrons would elements in group 18 make bonds?
No
Why do ionic bonds hold together?
The positive and negative ion attract each other
Water is made when oxygen is bonded to another element with covalent bonds. What is the other element and how many atoms of that element is needed?
two hydrogens
Metallic bonds give metals certain properties. One of the properties is ductility. What is ductility?
Ductility is the ability to be made into wires.
Would two positive ions make bonds?Why?
No, like charges repel
How many valence electrons does aluminum have?
3
What is the formula for Lead (IV) nitrate
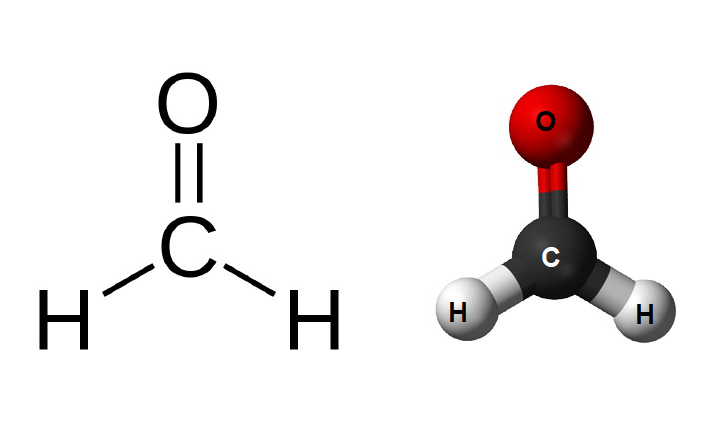
Draw the lewis dot of CH2O
Mixing two or more metals makes this.
An alloy
What is the charge of a neutral atom that loses 3 electrons?
3+
Why wont elements in group 18 make bonds?
they have 8 valence electrons
What are the qualities (give 2) of a substance that is bonded by ionic bonds?
crystal shape, high melting points and conduct electricity
What are the qualities ( give 2) of a substance that is bonded by covalent bonds?
Burn easy, low boiling point and poor conductors
List three properties of metals.