wavelenth, frequency, and energy
The part of the wave labeled #3 below.
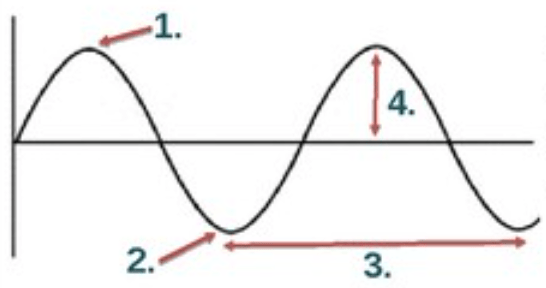
What is wavelength?
The specific bright lines emitted by elements as electrons fall from an exited to ground state is called this.
What is a line-emmision spectra?
The name for the lowest allowable energy state of an atom.
What is the ground state?
The number of valence electrons in all group 13 elements.
What is 3?
The speed of gamma rays.
What is 3.00 x 108 m/s (c; speed of light)?
ALL PLAY!
The longer the wavelength, the _______ the frequency.
What is lower?
A particle of light that carries a quantum of energy.
What is a photon?
What the principal quantum number tells you.
What is the main energy level?
ALL PLAY!
The full electron configuration for sulfur.
What is 1s2 2s2 2p6 3s2 3p4 ?
The Lewis dot structure for oxygen.
What is

What energy is associated with with the violet portion of the electromagnetic spectrum if it has a frequency of 7.2 x 1014 Hz?
What is 4.7 x 10-19 J?
ALL PLAY!!
The color of visible light with the lowest energy.
What is red?
When light is passed through a glass prism, the atomic emission spectrum is produced. Each element has it's own set of frequencies emitted by the atoms of the element. The element(s) in the mixture is(are) ______________.
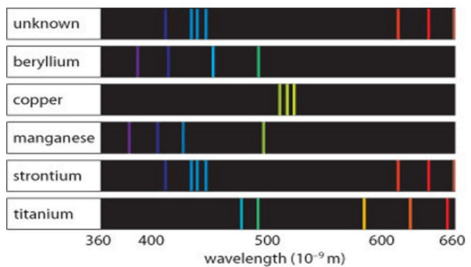
What is strontium?
The number of obitals for the s, p, d, and f sublevels.
What are 1, 3, 5, and 7?
The noble gas electron configuration for bromide ion (Br-1).
What is [Ar] 4s2 3d10 4p6 ?
ALL PLAY!
Electron in outermost orbital--typically associated with properties of the element and chemical bonding.
What is a valence electron?
The frequency of an x-ray with a wavelength of 2.6 x 10-10 m.
What is 1.15 x 1018 Hz
ALL PLAY!
Of radio waves, x-rays, infrared light, and orange light, the one with the largest frequency is ___________.

What is x-ray?
ALL PLAY!
The orbitals that are filling in Period 5.
What are the 5s, 4d, and 5p?
ALL PLAY!
The rule of electron filling violated here:
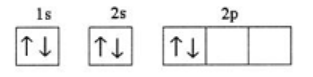
What is Hund's rule?
ALL PLAY!!
The noble-gas electron configuration for Rh.
What is [Kr] 5s2 4d7 ?
ALL PLAY!
The Lewis Dot structure for the element in period 3, group 14.
What is

The frequency of blue light with a wavelength of 480 nm.
What is 6.25 x 1014 Hz?
How wavelength and energy are related.
What is inversely proportional?
ALL PLAY!!
When atoms of an element emit light, it is because their electrons are doing this.
What is dropping from an exited (high) level to a lower (ground) level?
ALL PLAY!!
The total number of electrons that are in n=3. (Principle quantum number 3).
What is 18?
ALL PLAY!
This is the noble-gas electron configuration of arsenic with the valence electrons circled.
What is [Ar]4s23d104p3? (4s2 and 4p3 should be circled)
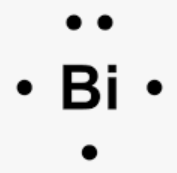
The number of valence electrons in this element AND its lewis dot diagram:
[Xe] 6s2 4f14 5d10 6p3
What is five and
The energy of a microwave with a wavelength of 15.0 cm.
What is 1.33 x 10-24 J?