Which two (ECM) are pure substances?
What are compounds and elements?
The capability of a substance to be molded and shaped or hammered into sheets without breaking is known as —
What is malleability?
Which ball is more dense than the water?
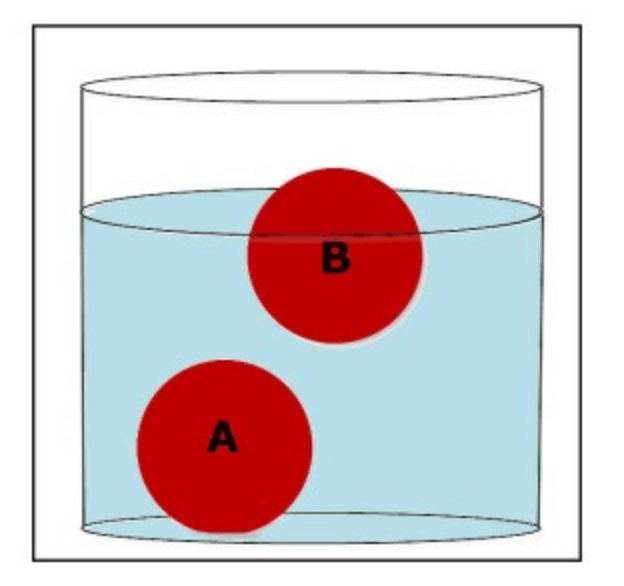
What is ball A?
Mixing silver nitrate and sodium chloride in water will cause silver chloride to precipitate out of solution. In this example, the precipitate is silver chloride.
Which example represents the formation of a precipitate?
A. A cut apple turns brown when left out in the open air.
B. Heat and light are produced when paper burns.
C. Gas is released when vinegar and baking soda are mixed together.
D. White chunks form when you add vinegar to milk or heavy cream.
What is D?
What is malleability?
What is the ability for a metal to be hammered or shaped into sheets?
How many capital letters do elements have?
What about compounds?
What is 1 and 2 or more?
Are metals high or low conductors?
Do metals have high or low malleability?
What is high?
What is high?
Which ball is less dense than the water?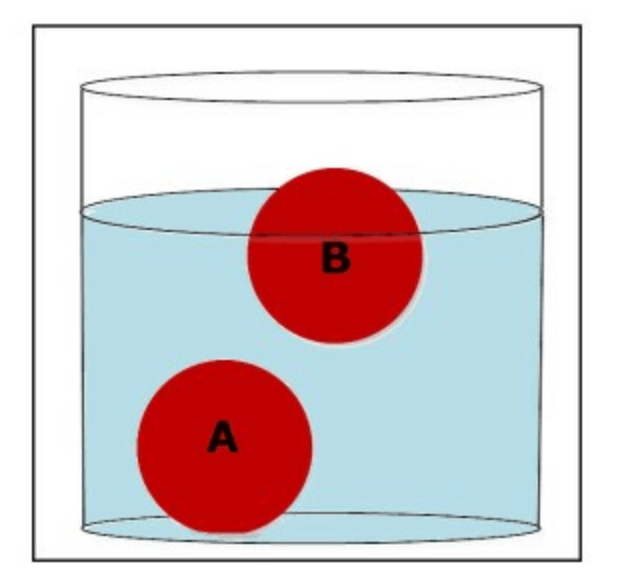
What is ball B?
When vinegar and baking soda are combined, bubbles and foam are produced. What evidence demonstrates that a chemical change has occurred?
What is bubbles? And/or What is a gas formed?
What is the luster of metalloids?
What is either shiny or dull?
Elements are represented by chemical _______.
What is symbols?
Do metals or nonmetals have high luster?
What is metals?
Calculate the density of a cube with a mass of 180 g and a volume of 9 mL.
What is 20 g/mL?
______ changes will always make a NEW substance.
What is chemical?
What is ductility?
What is the ability of a metal to be drawn into wires?
Compounds are represented by chemical _______.
What are formulas?
The capability of a substance to be stretched into thin wires without breaking is known as —
What is ductility?
Suzy found the volume of a key to be 10 mL and the mass to be 40 g. What is the keys density?
What is 4 g/mL?
Is state of matter change a chemical or physical change?
What is a physical change?
Give 3 properties of metals.
What is.....high melting point, shiny luster, malleable, ductile, and a conductor.
Which of the following is an element?
CO2
H2O
Cu
What is Cu?
Give me 3 properties of nonmetals.
What is...(answers will vary)?
A student needs to find the density of a cube. Each side of the cube measures 2 cm, and the mass of the cube is 16 g.
What is the approximate density of the cube?
What is 2 g/cm3?
What are the 5 indicators to a chemical change?
What is precipitate, energy change, color change, bubbles, and smell change?
If the mass of this cube is 12 g, what is the density? (hint: USE A CALCULATOR!)
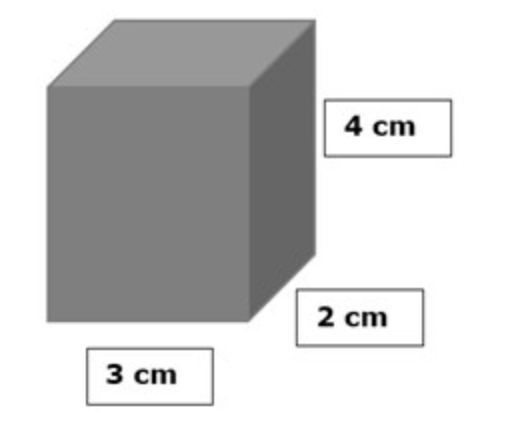
What is 0.5 g/cm3?