A mixture in which one substance is evenly distributed uniformly throughout another substance is a?
solution
 Chromatography is a process for separating components of a mixture. True or False?
Chromatography is a process for separating components of a mixture. True or False?
True
What is silver nitrate (AgNO₃) classified as?
Element
Compound
Mixture
Compound
A container is filled with a mixture. What should a student do to determine whether copper particles are in the mixture?
Magnet, boil, filter.
use a magnet
What is an example of a mixture?
Iron, copper, steel, sodium.
Steel is not a element, It is mainly iron and carbon.
Mixtures can be easily
a. melted
b. separated
c. destroyed
Seperated
:max_bytes(150000):strip_icc()/chemistry-distillation-58aef7d15f9b58a3c92007fa.jpg) What is a process of purifying a liquid compound by heating it into a vapor that is then condensed back into a liquid?
What is a process of purifying a liquid compound by heating it into a vapor that is then condensed back into a liquid?
Distillation
Coach Fawcett is making a kilt. It is made from lambs wool and has many different dyes to make it bright and colorful like his personality. What is the best way to separate the dyes?
Filtration, boiling, chromatography, distillation.
chromatography
What pure substance is composed of more than one element?
Hydrogen, oxygen, air, water
water
What is the odd one out?
Copper, aluminum, bronze, magnesium.
Bronze, it is a mixture of copper and tin.
Which of the following is an example of the formation of a mixture?
Rust forming on Moss's lawnmower
Salt dissolving in water
Sodium and chlorine forming salt
Salt dissolving in water
What type of mixture do you need to shake well and why would you need to shake it?
Suspensions.
To ensure it is evenly dosed.
Students are investigating the physical properties of three solid mixtures by placing each solid mixture in liquid water. What physical property are the students testing?
Boiling point, solvent strength, solubility.
solubility
Piper got some captain crunch cereal and pours a cup of milk onto the cereal. She decides it is not sweet enough and adds some honey. Yum, delicious! Is it a element, compound, or mixture?
Mixture, it has kept its original properties and is heterogenous.
Which substance is most likely classified as a colloid?
Soda, water, oxygen, fog.
Fog is a colloidal solution in which water (liquid, dispersed phase) is dispersed in the air (gas, dispersion medium)
Which of the following is an example of a container filled with a pure substance rather than with a mixture?
Balloon filled with air
Balloon filled with helium
Glass filled with water
Glass filled with tea
Balloon filled with helium
When Chemical X is added to a liquid, the chemical breaks into Substances Y and Z. It is not possible to break Substances Y and Z into simpler particles.
What substances are Y an Z?
Element, compound, mixture?
Element.
if you are making kool aid, what is the water?
Solution, solvent, solute.
solvent
Apple juice is what type of mixture?
Heterogenous
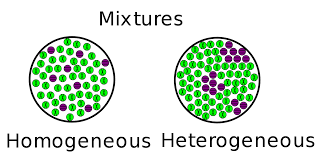
What makes a mixture heterogenous vs homogenous?
Non uniform particles, that are distinct from one another.
Students make create sand, gravel, and a coffee filter to separate the water from the particles within. What type of system are they using to seperate the water?
Filtration
What is the best method for separating a metallic silvery white substance from the liquid in the beaker?
Filtration
if you are making kool aid, what is the kool aid sugar?
Solution, solvent, solute.
solute
Milk is a homogenous mixture with large particles of one substance. What is this a example of?
Suspension, colloid, solvent
Colloid
Compounds are the basic building blocks necessary to form all matter.
True or false.
False, atoms (protons, neutrons, and electrons) in different combinations create all the diverity in the universe.
You will learn about them soon.