Energy that moves through pulsing (shaking) air particles.
What is sound energy?
Conner eats biscuits and gravy, then walks to school. This is an example of converting _______ energy to ________ energy.
What is chemical to kinetic?
What is the kinetic energy of a 1 kg soccer ball rolling with a speed of 1 m/s?
0.5 J
According to the 1st Law of Thermodynamics, energy cannot be _______ nor _______, only transferred.
created nor destroyed
According to the 2nd Law of Thermodynamics, heat always transfers from ________ to ________ when objects are in contact with each other.
hot to cold
Gravitational Potential Energy is based on the height, gravity acting upon it, and _______ of an object.
What is mass?
A microwave converts ________ energy to ________ energy (before reaching the food).
What is electrical to radiant?
A 2 kg box is lifted 10 m off the ground. How much more Gravitational Potential Energy would the box have if it were lifted to 15 m? Assume g = 9.8 m/s^2
98 J
One way to model the 1st Law of Thermodynamics is by showing that, in spite of changes in energy in a closed system, the total energy never changes. What is this model called?
Energy Pie Chart
Which type of energy is often developed as a result of friction and air resistance?
thermal energy
Also known as the "total average kinetic energy" of the particles in an object.
What is thermal energy?
What is the name of the special machine designed to perform a simple task with as many energy conversions as possible?
Rube Goldberg Machine
Jupiter's acceleration due to gravity is 24.79 m/s^2. If a 1 kg book was lifted 10 m off the ground, what would be the book's gravitational potential energy? Include units.
247.9 J
(or 247.9 (kg*m^2)/s^2)
If a hot cup of coffee has a total of 2100 J of energy after releasing 200 J of heat to its surroundings, what was the initial energy of the coffee?
2300 J
A car does 4500 J of work, while releasing 2000 J of heat to its surroundings. How much thermal energy was initially consumed by the engine?
6500 J
Energy of a compound that changes as bonds between its atoms are rearranged.
What is chemical energy?
In photosynthesis, a plant is converting both chemical and _________ energy into __________ energy.
What is radiant energy to chemical energy?
A roller coaster train undergoes a test-run, and travels with just enough speed to reach the top of a 30 m hill and stop. If the train has a mass of 4000 kg, what was the train's speed at the bottom of the hill? Ignore friction. g = 9.8 m/s^2
24.2 m/s
or √(588) m/s
Energy lost from a system to its surroundings is known as __________ energy.
dissipated
What is the work done by a boat that is 25% efficient and has an input of 200 J of thermal energy?
50 J
Energy that is used to bind the protons and neutrons in an atom together.
What is nuclear energy?
In a nuclear power plant, the initial input of energy is __________ energy, and the final output of energy (to the grid) is _______ energy.
What is nuclear to electrical?
A 50-kg crate that is falling has a terminal velocity of 40 m/s at an altitude of 100 m above the ground. Which is larger, the crate's Kinetic Energy or its Gravitational Potential Energy? Assume g = 9.8 m/s^2
Gravitational Potential Energy
Describe what is happening to this object without using the word, "energy."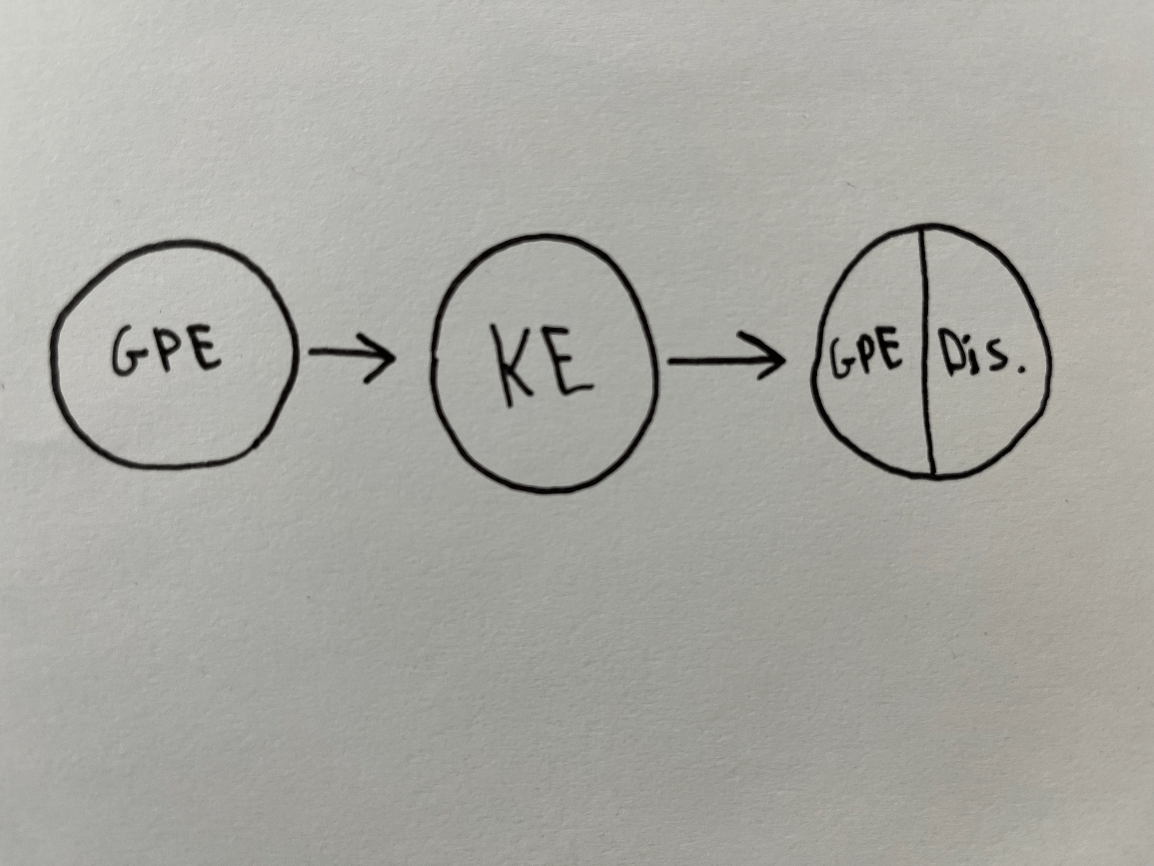
Possible example: The object fell, then rose after colliding with the ground.
What is the maximum efficiency an engine can have if it operates from a hot reservoir that is 600 K and a cold reservoir that is 150 K?
75%