Solar panels are devices that change light energy from the Sun into electrical energy. Solar panels can be used to power all kinds of things, from calculators to cars. A science class has built a solar-powered model car. How could the students best measure the amount of electrical energy produced by the solar panels that power the car?
Measure the current produced by the solar panels.
What is described as a device that converts chemical energy to electric energy?
A. battery
B. capacitor
C. lightbulb
D. resistor
battery
Temperature is the measure of the average
A. nuclear energy in all the nuclei in a sample.
B. chemical energy in all the chemical bonds in a sample.
C. kinetic energy of all the particles in a sample.
D. potential energy of all the particles in a sample
C. kinetic energy of all the particles in a sample.
What is taking place at the line labeled Y?
evaporation
How does the motion of the atoms in the spoon change when the spoon is placed in the hot coffee?
The atoms vibrate faster due to the conduction of heat through atoms in the spoon.
When the switch is set to "Off," which types of energy are present in the circuit?
electrical energy and chemical energy
Hydroelectric dams are often used to generate electrical energy. The energy of the water flowing over the dam is harnessed and transformed into electrical energy that can power appliances and other tools. What type of energy is transformed into electrical energy by hydroelectric dams?
Mechanical Energy
Temperature can be used to measure a particle's
A. chemical energy.
B. ionic energy.
C. kinetic energy.
D. potential energy.
C. kinetic energy.
Which can be concluded from Point G to H in the graph?
From time G to H, heat energy was absorbed.
Javier is designing a container to hold cold items. Which substance should Javier use to best insulate the container?
A. air, because it requires a lot of energy for air to change temperatures
B. copper, because if requires very little energy for copper to change temperatures
C. glass, because it requires a lot of energy for glass to change temperatures
D. iron, because it requires a very little energy for iron to change temperatures
A. air, because it requires a lot of energy for air to change temperatures
Amanda is watching a fireworks display with her father. She loves watching the different colors of light as the fireworks explode in the air. Most things that give off light give off energy in what other form as well?
heat energy
Which action involves the most efficient conversion of electrical energy into thermal energy?
A. a light bulb turning on inside an oven
B. a dishwasher rinsing dirty dishes with cold water
C.an electric mixer spinning to mix ingredients
D.an oven coil heating up to bake a cake
D.an oven coil heating up to bake a cake
when the kinetic energy of the particles in a sample of matter is lowered then its ........................ energy decreases
Kinetic
Why does temperature remain constant from when t = 2 min to when t = 5 min?
because heat energy is released from the substance as it changes from a liquid to a solid.
Henrietta wants to choose a metal that has the best rate of heat conduction so she can use it to make a new frying pan. Four metals and their specific heats are given in the table.
MetalsSpecific Heat(J/g°C)
aluminum 0.900
iron 0.444
nicke l0.440
copper 0.385
Which metal is the best choice for Henrietta's frying pan?
Copper
Which best describes energy changes in a system?
Energy is conserved, and it cannot be created or destroyed.
Computers can turn electrical energy into light and sound energy. Which other type of energy does a computer produce?
Thermal Energy
Which of the following energy transfer mechanisms is occurring between the warmer solid atoms and the cooler solid atoms as they collide with each other?
Conduction
Which of the following does the material experience during interval xy?
an increase in energy absorption and a phase change
Formula for Specific heat in Triangle
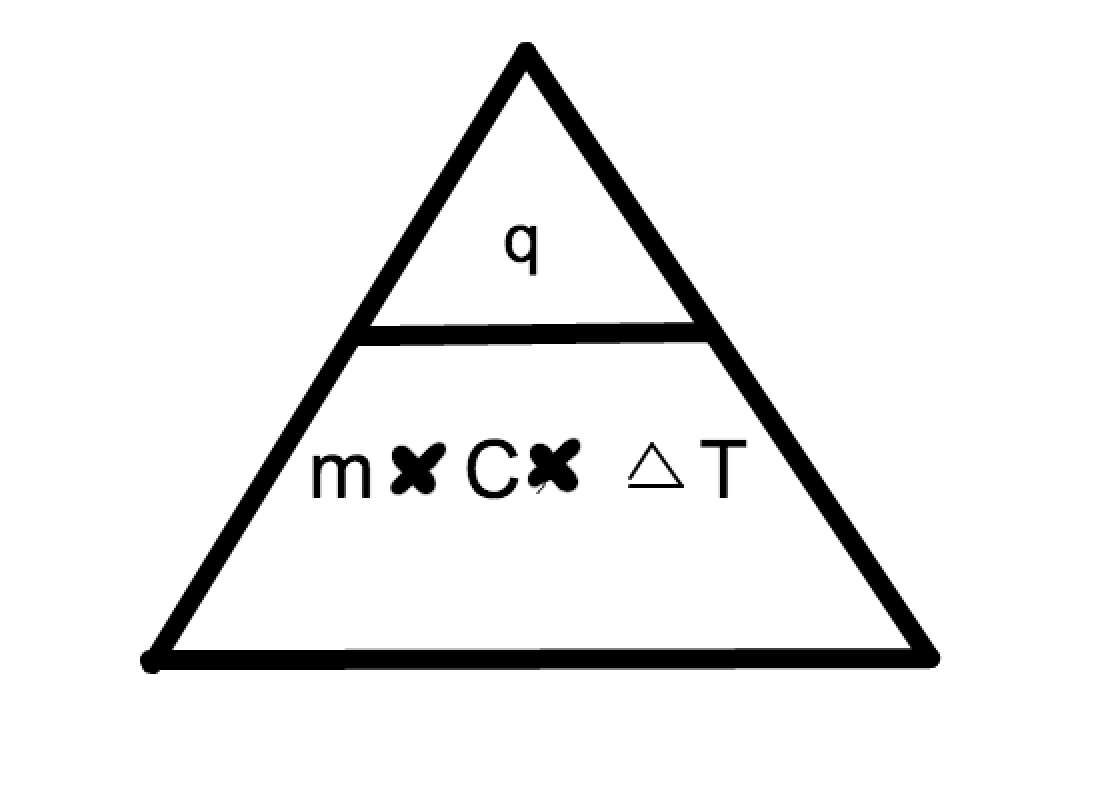
At point O, what is being brought into the home and sent back out to the power lines?
Electrical Energy
Unlike ordinary metal, working solar panels remain cool in strong sunlight. This suggests that the energy from sunlight hitting the panels is
Not absorbing Solar Energy
Air thermals are updrafts of warm air that rise from the ground into the sky. By flying in a spiral pattern birds are able to ride these air currents to higher altitudes.
What process enables birds to fly to higher altitudes without flapping their wings, while riding these air thermals?
convection because warm air rises and cool air sinks
Which point on the diagram corresponds to conditions in which the solid and gas phases are in equilibrium?
D
Definition for Specific Heat