Atomic Structure
Organizing The Periodic Table
The Element Families
classifying metals, non metals, and metalloid
100
A positively charged subatomic particle, with a symbol of "p" or "p+". It is located inside the nucleus and has an relative mass of 1 unit.
What are protons
100
Vertical columns that share the same number of valence electrons. first column has 1 valence electron, second column has 2, the third column has 3 etc..
What are groups
100
Shiny, malleable, metals which are highly reactive when exposed to air or water. Are in group one on the periodic table and become more reactive as the go down the column. are solid at room temperature. Ex: Lithium (Li), Sodium (Na), Potassium (K)
What are alkali metals
100
Are located in the yellow


Where are non metals
200
A subatomic particle with a neutral charge and a symbol of "n0". Is located in the nucleus and has a relative mass of 1 unit.
What are neutrons
200
Represents the number of protons in the nucleus. Increases by one as you go across the periodic table.
What is atomic number
200
This family is located in the red group
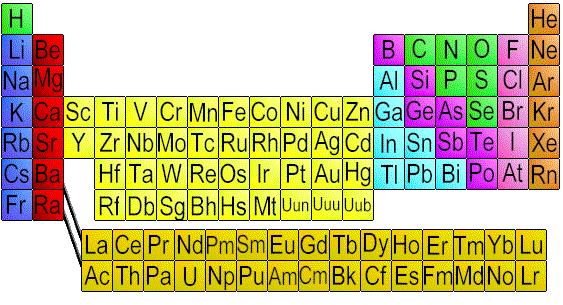

Where are alkali earth metals
200
Are located in the blue


Where are metalloids
300
A negatively charged subatomic particle with a symbol of
"e-". It orbits the nucleus in a planet like orbit called an electron shell and has 1/1800 the mass of a proton.
What are electrons
300
Horizontal rows that share the same number of electron shells. first row has 1 shell, second row has 2, third one has 3 etc.. goes all the way down until seven.
What are periods
300
Are the most stable and unreactive non metals. Never combine with other elements to make new substances. Are always gases at room temperature.
Ex: Helium (He), Neon (Ne), Argon (Ar)
What are noble gases
300
Located in groups 1-12 and some in groups 12-16. All of them are , shiny, ductile, malleable, and good conductors of electricity.
What are metals
400
An orbit around the nucleus which carries electrons.
What is electron shell
400
The number of electrons in the outer most shell.
What are valence electrons
400
Shiny, malleable, metals which are fairly reactive when exposed to air or water. Are located under group 2 of the periodic table and are solid at room temperature.
Ex: Beryllium (Be), Magnesium (Mg), Calcium (Ca)
What are alkali earth metals
400
Are located on a divider called the staircase which separates metals from non metas. to the right of them are non metals and to the left of them are metals. they have some properties in common with metals and some properties in common with non metals.
What are mettaliods
500
Is the positively charged centre of the atom which contains protons and neutrons. Takes up 99% of the atoms mass but only 1% of the atoms entire volume.
What is nucleus
500
Is equivalent to the total number of protons and neutrons inside the nucleus. Is always rounded to the nearest whole number.
What is atomic mass
500
Are located under group 17. they are the most reactive non metals in the periodic table and can combine with other elements to make new substances. can be solid, liquid, or gas at room temperature.
Ex: Fluorine (F), Chorine (Cl)
What are halogens
500
These element are located in groups 14-18. Are poor conductors of electricity, non ductile and non malleable. They also tend to be liquid or gas at room temperature.
What are non metals