What is the total energy content of a system called?
Enthalpy
The state of matter change from a gas to a liquid is called
Condensing
What is a physical or chemical change in which a system absorbs energy from its surroundings
Endothermic reaction
Draw the part of the graph that shows the activation energy
curve
When a substance is going from a liquid to a gas it is called
boiling
caloric content is
the energy that food has.
What is an exothermic reaction?
a physical or chemical change in which energy is released by a system to its surroundings

B
Define heat of combustion
the energy released as heat by the complete combustion of one mole of a substance
Define heat of solution
the amount of heat energy absorbed or released when a specific amount of solute dissolves in a solvent
Which type or reaction has products with more energy than the reactants?
endothermic

B
What are the differences between warming and coolling
cooling is heat leaving a system, warming is heat entering a system
Describe the differences between latent and sensible heat
latent-heat that doesn't raise the temperature of the object. only happens during a phase change
sensible-temperature rises, doesn't happen during a phase change.
Which reaction has a higher activation energy?
endothermic?
Where would the sensible heat be in this picture?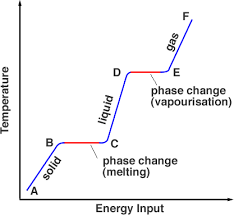
C-D, a-b, e-f
describe the difference between heat and temperature
temperature is the measure of kinetic energy in the molecules
heat is the kinetic energy in the molecules
Describe how the enthalpy change and the heat of reaction are related based on their definitions.
the heat of reaction shows how much the kinetic energy of the atoms has changed throughout the reaction and the enthalpy change tells you the total change in energy throughout the reaction. This is the same number.
How does the activation energy relate to the stability of the products. Describe which graph has more stable products.
exothermic
 Describe what part of the graph would be the latent heat and why
Describe what part of the graph would be the latent heat and why
b-c phase change, d-e phase change