Matter and Motion
Atomic Structure
Periodic Table & Bonding
Solutions
Energy
100
According to Boyle's Law, as pressure increases, volume ________.
What is decreases?
100
All atoms of an element have the same number of __________ which identifies the element.
What is protons?
100
The left side of the periodic table has __________ and they form _________ ions (cations)
What is metals; positive?
100
A substance with a pH below 7
What is an acid?
100
Energy of motion.
What is kinetic energy?
200
A 10 cm cube has a mass of 500 g, what is the density of the cube?
What is 0.5 g per cubic centimeters?
200
An electron is a particle with a __________ charge, found ________ the nucleus.
What is negative; outside/orbiting?
200
These elements have a full octet and are therefore stable.
What is the noble gases/8A/18?
200
Oven cleaner, tastes bitter, has hydroxide ions and turns litmus paper blue. Oven cleaner is classified as ____________.
What is a base?
200
Energy from the sun reaches the earth through __________.
What is radiation?
300
If a gas, under constant pressure, with a volume of 3 liters is heated, its new volume will be:
1. More than 3 liters
2. Less than 3 liters
3. The same as it was before heating
What is 1. More than 3 liters?
300
Carbon-13 and Carbon-14 have the same number of ________ but different number of __________ because they are __________.
What is protons; neutrons; isotopes.
300
The name of LiO2
What is Lithium oxide?
300
What 3 factors can increase the solubility of a solute in a solution?
What is increasing temperature of solvent; breaking solute in smaller pieces (increase surface area); stirring; increasing the concentration of the solvent;
300
In a demonstration of heating by convection, your teacher heats a beaker of water to boiling on a hot plate. Which hypothesis correctly describes how heating by convection occurs?
A. As particles of a substance are heated, energy is
transferred as more energetic particles within the
substance move from one place to another.
B. As particles of a substance are heated, energy is
transferred by gas bubbles within a liquid.
C. As particles of a substance are heated, energy is
transferred by electromagnetic waves.
What is A?
400
The two part classification for glucose (C6H12O6) is ___________, _____________.
What is pure substance, compound?
400
How many neutrons are in Phosphorus-33?
What is 18?
400
NO is formed through a _________ bond and is called ________.
What is covalent; Nitrogen monoxide?
400
In a saltwater solution, _____________ solute and _____________ is the solvent.
What is salt; water.
400
A battery provides _______ ENERGY.
What is chemical?
500
When water boils, bubbles are produced. This is an example of a ______________ change.
What is physical?
500
The neutral Lithium atom has _________ mass compared to the Lithium ion and ________ electrons.
What is the same; more
500
A _____________ reaction occurs when Al2O3 produces aluminum and oxygen. And the coefficents to balance the equation are ___, ____, ____.
What is Decomposition; 2,4,3.
500
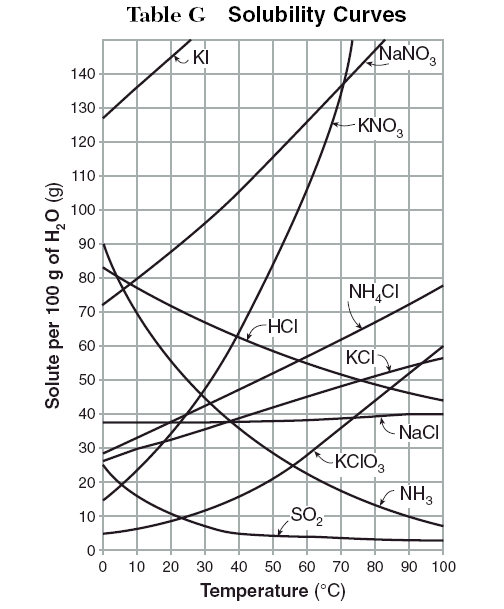 The solubility of potassium chloride at 10 degrees Celsius.
The solubility of potassium chloride at 10 degrees Celsius.What is 30 grams?
500
How much heat must be absorbed by 375 grams of water to raise its temperature by 25° C? Specific heat of water = 4.18 J/g° C.
What is 39,187.5 J?