Is this equation an endothermic or Exothermic reaction?
NaOH + HCI → NaCI + H2O ΔH= +122kj
endothermic
The following reaction is allowed to reach equilibrium.
COBr2(g)⇌CO(g)+Br2(g)
What happens to the reaction when inert argon gas, Ar(g), is added?
Nothing happens/ reaction still at equilibrium.
What disturbance likely occurred at time (t). 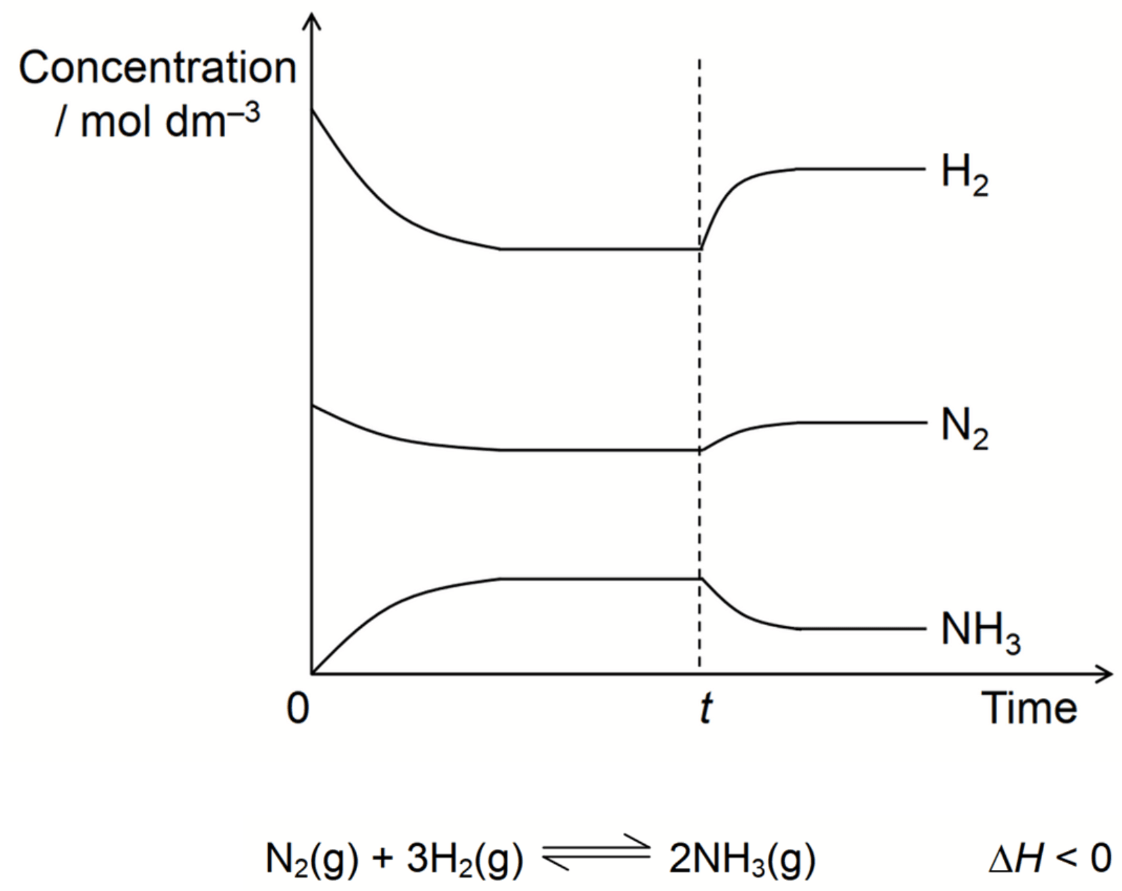
Temperature decrease
What is the Keq equation for
N2(g) +3H2(g) <—> 2NH3(g)
Keq = [NH3]2 /[N2][H2]3
This AI chatbot took the internet by storm in late 2022, captivating users with its ability to generate human-quality text.
Chat GPT
What is the effect of adding a catalyst to a reaction in equilibrium?
It increases the forward and reverse reactions equally, and thus doesn’t alter the position of equilibrium.
The following exothermic reaction is allowed to reach equilibrium.
2H2(g)+O2(g) ⇌ 2H2O(g) + HEAT
What happens to the reaction when the temperature is decreased?
Shift right - Favors forward reaction
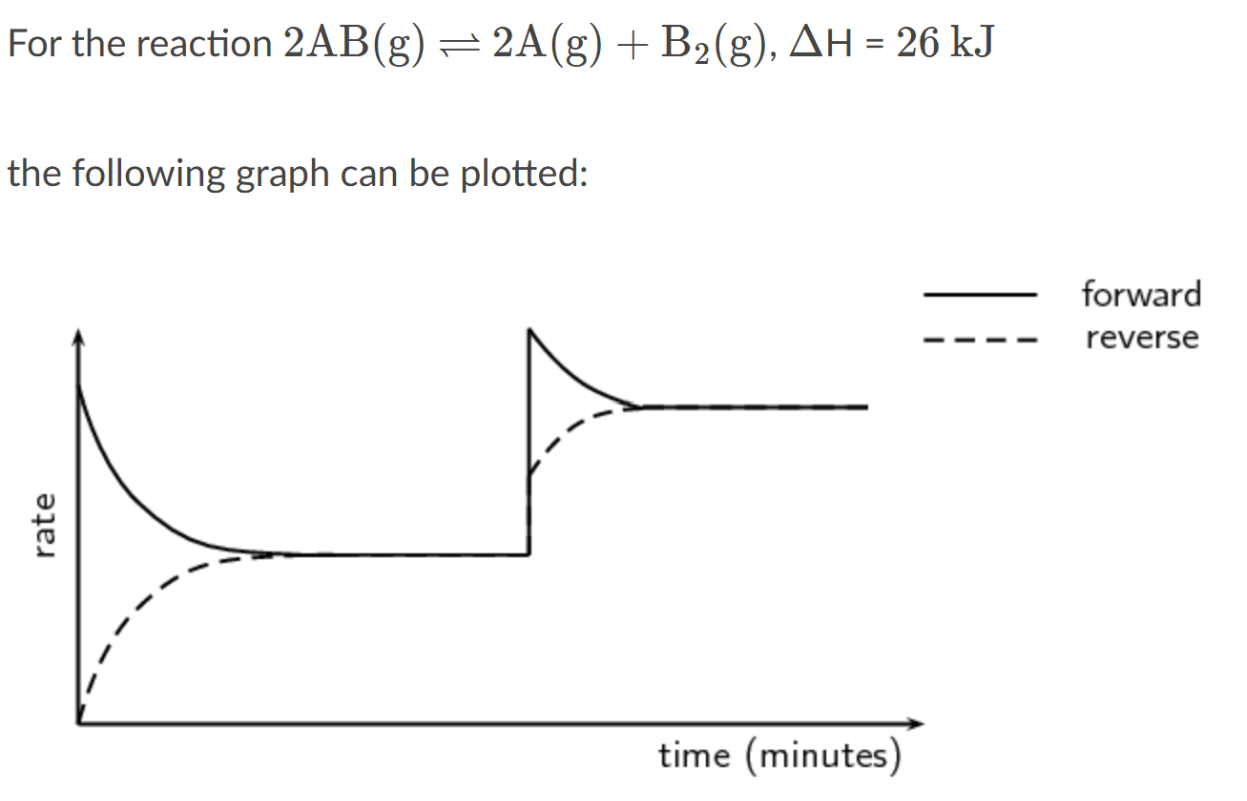
What stress has likely occurred in this system?
Temperature increase
When Keq > 1 which side of the reaction is favoured?
Products/Right Side
This highly anticipated video game, a sequel to a beloved franchise, was released in 2023 and broke sales records.
The Legend of Zelda: Tears of the Kingdom?

Consider the reaction:
A(g) + B(g) ⇋ C(g)
In an equilibrium mixture the following concentrations were found:
[A] = 0.45M, [B] = 0.63M and [C] = 0.30M.
Calculate the value of the equilibrium constant for this reaction.
Keq = 1.1
What is the affect of adding a catalyst to an equilibrium reaction?
Increases the forward and reverse reactions equally, meaning no change in the position of equilibrium

N2(g)+3H2(g)⇌2NH3(g) ΔH=−92kJmol−1
What happened at t1?
N2 gas was added
What is the equilibrium constant K for the chemical equation:
ClNO2(g)+NO(g)<—>NO2(g)+ClNO(g)
K=[NO2][ClNO]/[ClNO2][NO]
This princess, known for her love of books and strong-willed personality, is from the kingdom of Arendelle.
Elsa
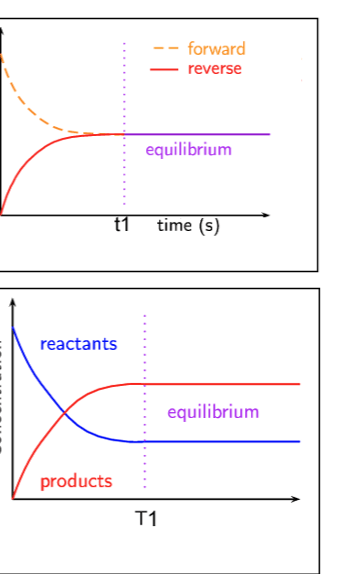 Both of these graphs show a reaction at equilibrium. For each of the graphs, how would we label the y axis?
Both of these graphs show a reaction at equilibrium. For each of the graphs, how would we label the y axis?
Top Graph - Rate
Bottom Graph - Concentration
Consider the equation:
BaCl2 (aq) + NaSO4 (aq) <--> 2NaCl (aq) + BaSO4 (s)
If barium chloride (BaCl2) is added to the system, what change occurs?
The reaction shifts to the right, which results to an increase in the yield of products.

N2(g)+3H2(g)⇌2NH3(g) ΔH=−92kJmol−1
What happened at t2?
Volume was decreased (increase in pressure)
What does it mean when a chemical reaction has an equilibrium constant of 173?
Mixture contains mostly products
This term refers to a specific aesthetic style that often involves vibrant colors, playful patterns, and nostalgic references.

Y2K
For the homogeneous gaseous reaction
A quantity of PCl5 was heated in a 1.0 L container at 250°C. At equilibrium, the gases were present in the following concentrations: PCl5 = 7.05 M, PCl3 = 0.54 M, Cl2 = 0.54 M. Calculate the value of the equilibrium constant for the dissociation of PCl5 at 250°C.
Keq = 0.041
What change would be expected to drive this reaction to the left?
NO2 (g) <--> 2NO2 (g)
An increase in pressure. This is due to how for every mole of reactant, there are 2 moles of product made.

N2(g)+3H2(g)⇌2NH3(g) ΔH=−92kJmol−1
What happened at t3?
Increase in Temperature
Consider the following reaction
A2(g) + B2(g) ⇋ 2AB(g)
If, at equilibrium, the concentrations are as follows: [A2] = 3.45 M, [B2] = 5.67 M and [AB] = 0.67 M
Find the value of the equilibrium constant, Keq
Keq = 0.023
Who is the highest paid athlete in 2024?
Cristiano Ronaldo