This tool is used to measure the mass of an object.
scale or balance
This pure substance is made of only one type of atom, and cannot be broken down.
Element
This separation technique can be used to separate water from undissolved sand or rocks.
Filtration (distillation and evaporation can also work!)
18. Which of the following cannot be decomposed through a chemical change?
(1) Lead (Pb)
(2) carbon dioxide (CO2)
(3) water (H2O)
(4) sugar (C6H12O6)
(1) Lead (Pb)
Lead cannot be decomposed because it is only one element.
How many significant figures are in 3300?
2 significant figures.
this can be used to measure volume very accurately (to the milliliter).
graduated cylinder
(beakers aren't accurate for volume).
You cannot see the different parts in this type of mixture.
Homogeneous Mixture
This separation technique can remove dissolved salt from water.
Evaporation (distillation can work too)
13. Which list of formulas represents elements, only?
CO2, H2O, NH3
H2, N2, O2
H2, Ne, NaCl
MgO, NaCl, O2
These each only have ONE type of atom.
How many significant figures are in 300.3?
4 significant figures.
This is the metric prefix with the largest factor in the Chemistry Reference table.
Kilo-
(this is the prefix).
You CAN see the parts in this type of mixture.
Heterogeneous Mixture
This method can be used to remove alcohol from water (two liquids with different boiling temperature).
Distillation.
Which is a characteristic of all mixtures?
They are homogeneous
They are heterogeneous
Their composition is in a definite ratio.
Their composition can be varied
How many significant figures are in 0.000330?
3 significant figures.
Based on the Reference Table, write down the density of element 80, Mercury (Hg) WITH THE CORRECT UNITS FOR DENSITY.
13.534 g/cm3
(YOU NEED THE UNITS)
This substance is made of two or more elements.
Which separation technique is used to separate colored pigments from each other using paper?
Chromatography.
A large sample of solid calcium sulfate is crushed into smaller pieces for testing. Which physical property is the same for the large and small pieces of calcium sulfate?
Mass
volume
density
Reaction rate
The density of a substance never changes!
Round 2245 to 2 significant figures.
2200
Rewrite the density formula so that you are solving for VOLUME instead of density.
v = ?
v = m/d
This type of matter can be separated using physical changes- using the separating techniques we learned in class.
Mixture.
Which separation technique is used to separate a mixture of two liquids with different boiling points?
distillation
11. Equal amounts of ethanol and water are mixed at room temperature. Which process is used to separate ethanol from the mixture? [reminder: ethanol is a liquid]
Distillation
Reduction
Filtration
Ionization
3. Distillation (separates two liquids)
Round 0.003452 to 2 significant figures.
0.0035
You have a very large piece of iron and a very small piece of iron. What property is probably the same?
(1) mass (2) volume
(3) density (4) number of atoms
(3) density
Density of a substance NEVER changes!
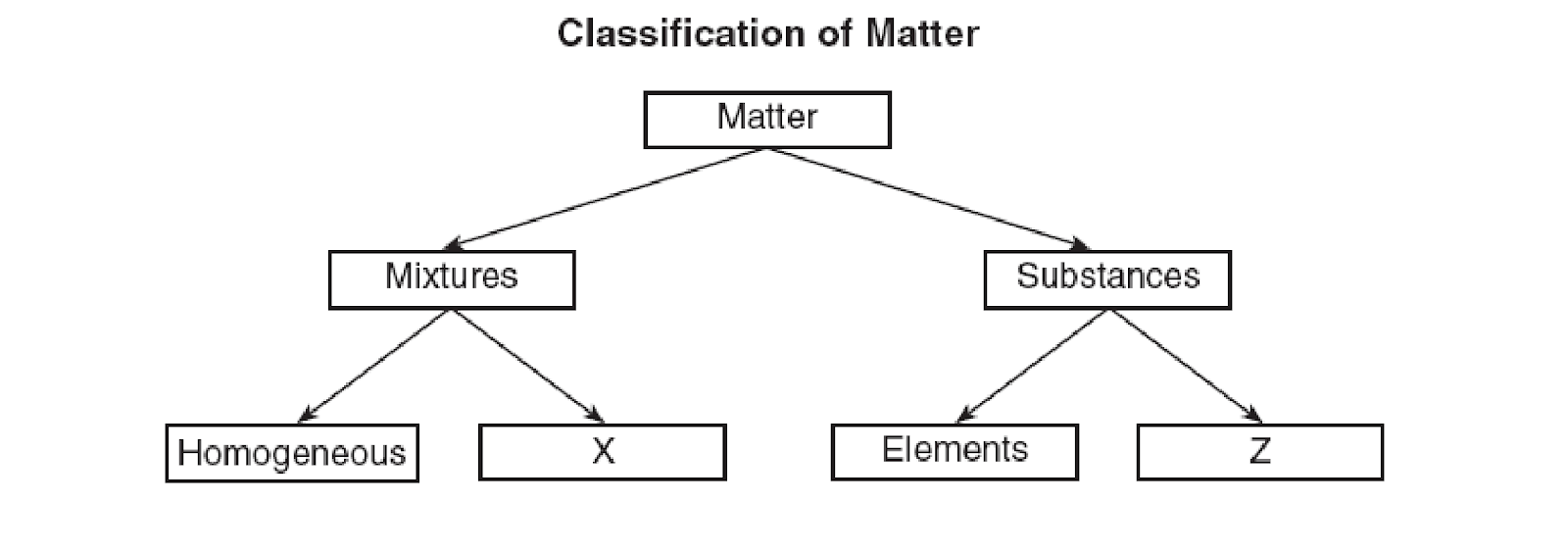 Which types of matter fill in X and Z?
Which types of matter fill in X and Z?
X = heterogeneous
Z = compounds
When Sample X is passed through a filter paper, a white solid, Y, remains on the paper and a clear liquid, Z, passes through. When liquid Z is evaporated, another white solid remains.
Sample X is best classified as...
an element
a compound
a heterogeneous mixture
a homogeneous mixture
3. a heterogeneous mixture
(the filter removed a visible solid, Y, from the sample X).
Sugar in water is classified as...
Element
Compound
Mixture because the proportion of its components is fixed
Mixture because the proportion of its components can vary
4. Mixture because the proportion of its components can vary.
DAILY DOUBLE (1200 pts)
Find the mass of a 1.0 cm3 sample of Copper, rounded to the appropriate number of SIG FIGS.
9.0 g
m = V*d = (1.0g)*(8.96 g/cm3) = 8.96 g
round to LEAST NUMBER OF SIG FIGS --> 9.0 g