Define: Theory
- a well-supported testable explanation of phenomena thathas occurred in the natural world
Differentiate between a polar covalent bond and a nonpolar covalent bond. Using the words:
• electronegativity
• unequal and equal
• sharing
- polar covalent bond: two atoms; one with a higher ELECTRONEGATIVITY; resulting in UNEQUAL SHARING of electrons between the two
- nonpolar covalent bond: two atoms; fairly equal ELECTRONEGATIVITY; resulting in EQUAL SHARING of electrons between the two
List out the inter vs intra-molecular bonds.
a. Which bonds hold together ONE molecule?
INTERmolecular bonds:
- Hydrogen bonds
- Van Der Waal's interactions
- Hydrophobic effect
INTRAmolecular bonds:
- Covalent bonds
- Ionic bonds
a. Intramolecular bonds hold together ONE molecule.
Define:
H+
OH-
[ ]
Acid
Base
Buffer
- Hydrogen ion/proton
- Hydroxide ion
- Concentration of
- a substance that donates H+ (protons)
- a substance that accepts H+ (protons)
- a substance that resists the change of pH (consisting of weak acid-base pairs)
Why is carbon the most significant element in regards to forming large and complex molecules?
- carbon can form four bonds
- carbon forms covalent bonds
Evolution is proven to be true because of what three things?
- Direct observations
- Homology
- Fossils
Distinguish between ionic and covalent chemical bonds.
Ionic bond: TRANSFER electrons (cation; anion)
Covalent bond: SHARING electrons (polar; nonpolar)
Bonus- are these bonds polar or nonpolar?
O-H
O-O
C-H
How do the number of H-bonds between water molecules determine the state of water?
- 0 bonds = gas
- 1,2,3 bonds = liquid
- 4 bonds = solid
How can we recognize acids and bases in chemical reactions?
Compare the reactants to the products:
Which one lost H+?
Which one gained H+?
Contrast saturated vs. unsaturated hydrocarbon chains.
Saturated hydrocarbon chains contain the maximum number of hydrogens (NO double bonds between carbon).
Unsaturated hydrocarbon chains do not have the maximum number of hydrogen (DO have double bonds between carbon).
What is the MAIN driving force of evolution?
(what allows evolution to be?)
Natural selection: those with favorable traits survive and pass on those traits to newer generations
AKA: species show evidence of “descent with modification” from common ancestors
What role do electrons/valence shells play in chemical bonds?
- electrons dictate an atom's reactivity (whether it will look to form bonds with other atoms)
Bonus- when are atoms reactive? when are they not?
What makes a molecule have polarity?
a. What type of bond creates polarity?
- having partial opposite charges due to a higher electronegativity from one atom relative to the other atom in the molecule
- polar covalent bonds
Explain how this (very significant) buffer works.
Board discussion.
What are the 4 ways that carbon "skeletons" can vary?
1. Length
2. Double bond positioning
3. Branching/linear
4. Presence of rings
What are the THREE domains of life?
a) State whether the organisms that fall into each domain are prokaryotic or eukaryotic.
1. Eukarya (eukaryotic)
2. Bacteria (prokaryotic)
3. Archaea (prokaryotic)
Identify the [6] major elements found in living organisms.
a) Determine which ones are strong and which ones are weak.
1. Sulfur (weak)
2. Phosphorus (weak)
3. Oxygen (strong)
4. Nitrogen (strong)
5. Carbon (weak)
6. Hydrogen (weak)
List and explain the four properties of water due to its ability to form hydrogen bonds.
1. High specific heat
2. Cohesion/adhesion
3. Universal solvent
4. Expansion upon freezing
Compare the pH of 14 to a pH of 10. Which is more acidic and by how much more?
- 10 is more acidic
- 10,000x more acidic
Today we have learned that the macromolecules inside of our body are built by molecules called monomers.
a. If I want to link one monomer to another monomer, what reaction is used?
b. If I want to break a bond between two monomers, what reaction is used?
a. Dehydration reaction
*bonus question: two or more monomers linked together is called a....
b. Hydrolysis
Name and classify the SIX kingdoms of life.
1. Bacteria
2. Archaea
3. Protists
4. Plantae
5. Fungi
6. Animalia
Distinguish between the following pairs of terms: neutron, electron, proton, atomic number, atomic mass/weight.
- neutron: subatomic particle with a neutral charge
- electron: subatomic particle with a negative charge
- proton: subatomic particle with a positive charge
- atomic number: # of protons
- atomic mass: # of protons +# of neutrons
Distinguish: hydrophobic and hydrophilic substances; a solute, a solvent, and a solution.
- hydrophobic: water-fearing (substances that are neutral/nonpolar)
-hydrophilic: water-loving (substances with partial opposite charges/polar)
-solute: substance that is being dissolved
-solvent: dissolving agent
-solution: 2 or more substances mixed together
- If the [OH-] = 1 x 10-2 M, then:
[H+] =
pH =
- If the [H+] = 1 x 10-10 M, then:
[OH-] =
pH =
- Given the pH of 3, what is [H+] and [OH-]?
1.
[H+] = 1 x 10-12
pH =12
2.
[OH-] = 1 x 10-4
pH = 10
Name the functional groups below.
a) Describe their properties
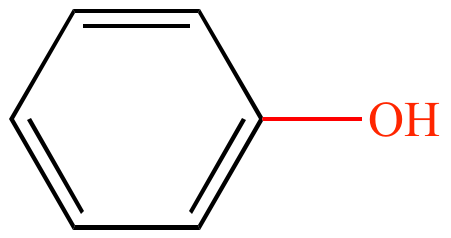

1. Hydroxyl Group (polar; hydrophilic)
2. Carboxyl Group (polar; hydrophilic)
3. Amino Group (polar; hydrophilic), Carbonyl (polar; hydrophilic)