How many Sig Figs is 3467?
4
Jack was directed to weigh a 550 g mass on the balance. After diligently goofing off for ten minutes, he quickly weighed the object and reported 558 g. What is the percent error?
1.5%
What is the density of a mineral if 427 g of the mineral occupy a volume of 35.0 mL.
12.2g/mL
Convert into Scientific Notation:
6894
6.894 x 103
3.4mm=_______cm
0.34cm
What is the metric unit for mass?
grams (g)
How many Sig Figs is 50.8?
3
Sean was told to measure 23 cm of copper wire to use in an experiment. Since his ruler only measured to 21 cm he used this amount of wire and his experiment didn’t go as planned. What is the percent error?
-8.7%
What is the mass of 25.0 cm3 of gold?
483g
Convert into Scientific Notation:
0.00342
3.42 x 10-3
234.45hL=________L
23445L
What Table on your Regents Chemistry Reference Table has both the equations for percent error and density?
Table T
How many Sig Figs is 0.00976?
3
Manny was assigned to determine the density of a sample of nickel metal. When he finished, he reported the density of nickel as 6.19 g/ml. However, Charlotte knew the density of nickel was 6.44 g/ml. What is the percent error?
-3.88%
What mass of Hg will occupy a volume of 50.0 mL?
678g
Convert into Scientific Notation:
98,500
9.85 x 104
34g=_________mg
34000mg
What is this called?

Graduated Cylinder
How many Sig Figs is 55,060,000?
4
An experiment to determine the volume of a "mole" of a gas was assigned to Ben. He didn't read the experiment carefully and concluded the volume was 18.7 liters. Lily knew he should have obtained 22.4 liters. What is the percent error?
-16.5%
A sample of metal has a volume of 45 cm3 and a mass of 121.5 g. Find the density of this sample and use Table S to identify this substance.
2.7g/cm3
Aluminum
Convert into an ordinary number:
6.540 x 107
65,400,000
0.0345L=__________cL
3.45cL
What is the measurement reading?
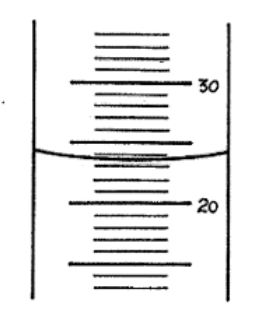
23.5 mL
How many Sig Figs is 0.000000000070800?
Ella measured the volume of her soda before she drank it for her lunch. She measured the volume of the 8 oz. bottle to be 11 oz. What is the percent error?
37.5%
Mercury (13.550g/mL), water (1.000g/mL), carbon tetrachloride (1.595g/mL), and cottonseed oil (0.926g/mL) are placed in a graduated cylinder and layers formed. Identity the order of layers from top to bottom.
Cotton Oil, Water, Carbon Tetrachloride, Mercury
Convert into an ordinary number:
8.5 x 10-8
0.000000085
34.456Km=___________mm
34456000mm
What is the hottest part of the bunsen burner flame?
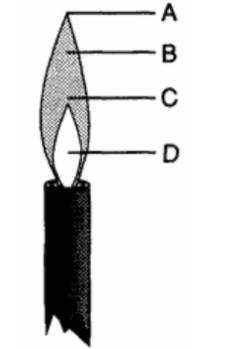
C